Advertisements
Advertisements
Question
Identify the gas evolved when sulphur is treated with concentrated nitric acid
Solution
When sulphur is treated with conc. nitric acid
APPEARS IN
RELATED QUESTIONS
Give a balanced chemical equation for Laboratory preparation of Nitric acid.
Ammonia is used in the Ostwald process.
Give the sources of reactants used in this process.
Give two chemical equations for the following:
Nitric acid showing as acidic character.
Give the chemical name and formula of the substance formed as a brown ring in the test for nitrate radical.
Mention three important uses of nitric acid. Give the property of nitric acid involved in the use.
Name the Following:
Gas obtained by treating manganese with 1% nirtric acid.
Choose the correct answer from the option given below :
Concentrated nitric acid oxidises phosphorus to :
Explain, why only all glass apparatus should be used for the preparation of nitric acid by heating concentrated sulphuric acid and potassium nitrate.
Give one test to distinguish between the following pair of chemicals.
Zinc nitrate solution and calcium nitrate solution.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid.
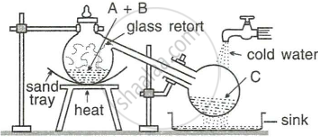
- Name A (a liquid), B (a solid), and C (a liquid). (Do not give the formulae).
- Write an equation to show how nitric acid undergoes decomposition.
- Write the equation for the reaction in which copper is oxidised by concentrated nitric acid.
