Advertisements
Advertisements
Question
Identify the monomer in the following polymeric structures.
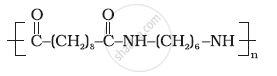
Solution
The monomers of the given polymeric structure are decanoic acid `[HOOC - (CH_2)_s-COOH]` and hexamethylene diamine `[H_2N(CH_2)_6 NH_2]`
APPEARS IN
RELATED QUESTIONS
What is natural rubber?
(A) Cis-1,4-polyisoprene
(B) Neoprene
(C) Trans-1,4-polyisoprene
(D) Butyl rubber
Write names and chemical formulae of monomers used in preparing Buna-S.
What is the role of sulphur in the vulcanisation of rubber?
How does the presence of double bonds in rubber molecules influence their structure and reactivity?
Write names and chemical formulae of monomers used in preparing Buna-N.
Write the formulae of the raw materials used for preparation of Buna-S
Write the structures of monomers used the following polymers:
Buna S
What is the role of Sulphur in the vulcanization of rubber?
Vulcanisation makes rubber:
(i) more elastic
(ii) soluble in inorganic solvent
(iii) crystalline
(iv) more stiff
Identify the polymer given below:
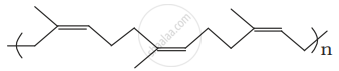
The polymer of natural rubber is:-
Ebonite is:-
Which of the following is correct regarding the drawbacks of raw rubber.
Match List I with List II.
| List I | List II |
| (Monomer Unit) | (Polymer) |
| (a) Caprolactum | (i) Natural rubber |
| (b) 2-Chloro-1, 3-butadiene | (ii) Buna-N |
| (c) Isoprene | (iii) Nylon-6 |
| (d) Acrylonitrile | (iv) Neoprene |
Choose the correct answer from the options given below:
