Advertisements
Advertisements
Question
If the charge on an electron is 1.6 × 10−19 coulombs, how many electrons should pass through a conductor in 1 second to constitute 1 ampere current?
Solution
Given:
Current,/=1A
Time,t=1s
Using the formula:
`I=Q/t`
or,
`Q=Ixxt `
=`1xx1`
=1=C
Now, if the charge is `1.6xx10^-19`C, the number of electrons is 1.So, if the charge is 1C, the number of electrons is given by:
`1/(1.6xx10^-19)xx1=6.25xx10^18`
Thus, `6.25xx10^18`electrons should pass though a conductor in 1 second to constitute 1 ampere current.
APPEARS IN
RELATED QUESTIONS
What is the unit of electric current?
State three factors on which the heat produced by an electric current depends. How does it depend on these factors?
The following circuit diagram shows three resistors 2 Ω, 4 Ω and R Ω connected to a battery of e.m.f 2V and internal resistance 3 Ω. If the main current of 0.25 A flows through the circuit, find:
- the p.d. across the 4 Ω resistors,
- the p.d. across the internal resistance of the cell,
- the p.d. across the R Ω or 2 Ω resistors, and
- the value of R.

The diagram shows two ways of connecting three lamps P, Q and R to A.C. supply of 220 V.
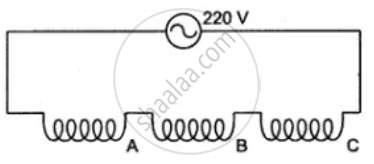
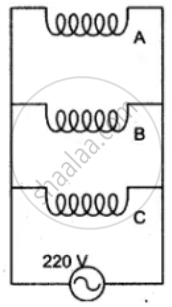
State the S.I. units of electrical power.
Define the term kilowatt - hour and state its value in S.I. unit.
An electric iron is rated 750 W, 230 V. Calculate the electrical energy consumed by the press in 16 hours.
