Advertisements
Advertisements
Question
If ‘X’ g. of potassium nitrate is added to 100 g. of water at 60°C and the salt dissolves completely then :
(a) is ‘X’ g. the solubility of potassium nitrate at 60°C.
(b) is the solution formed – saturated or unsaturated
(c) if on addition ‘X’ + ‘Y’ g. of potassium nitrate to the same amount of water at the same temperature and the solute now just remains behind after stirring then –
(d) is the solution now – saturated or unsaturated
(e) is ‘X’ + ‘Y’ g. the solubility of potassium nitrate.
Solution
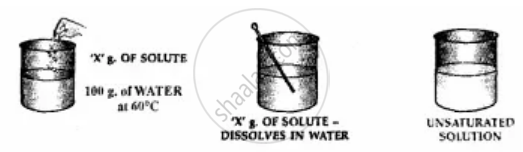
Add ‘X’ g. of solute i.e. potassium nitrate to 100 g. of water 60°C.
1. Stir the solute i.e. potassium nitrate in water thoroughly.
2. ‘X’ g. of the solute completely dissolves in water.
3. Add more solute and again stir thoroughly.
4. The solute continues to dissolves.
5. Water i.e. the solvent can dissolve more of the solute at the given temperature.
6. The solution is, therefore, is said to be unsaturated.
Add more solute to water till on adding an amount ‘X + Y’ g. of the solute i.e. potassium nitrate to 100 g. of water at 60°C.
1. The solute just remains behind after stirring.
2. The solution is now saturated.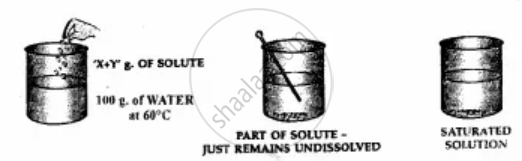
A saturated solution cannot dissolve more of the solute at a given temperature.
RELATED QUESTIONS
Why is water called a universal solvent?
Define:
Unsaturated solutions
Complete the statement by filling in the blanks with the correct words.
If potassium nitrate is added to water in a beaker to give a homogeneous mixture, then potassium nitrate is referred to as the __________, water as the __________, and the homogeneous mixture as the _______.
(solution/ solute/solvent/saturated solution)
Draw a neat labelled diagram of the addition of copper sulphate to water. Label solute, solvent, and solution in the same.
From the following substances given below state which will form a solution in the same.
(a) sodium carbonate
(b) calcium carbonate
(c) charcoal powder
(d) sodium sulphate
(e) table salt
(f) powdered particles of lead from a lead pencil
(g) iron powder
(h) copper filings
(i) sand particles
(j) honey
Define the term – solute
Define the term – solvent
Polar compounds are soluble in _____ solvents
Solutions which contain three components are called a binary solution.
A homogeneous mixture of two or more substances is called ______.
