Advertisements
Advertisements
Question
In a coordination entity of the type [PtCl2(en)2]2+ which isomer will show optical isomerism?
Options
cis-isomer
trans-isomer
fac-isomer
mer-isomer
MCQ
Solution
cis-isomer
Explanation:
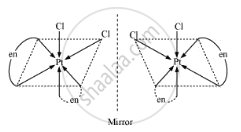
We can see that in the cis isomer the mirror images are non-superimposable whereas in trans-isomer the mirror images will be superimposable and hence will not show optical activity.
shaalaa.com
Is there an error in this question or solution?
