Advertisements
Advertisements
Question
In ozone molecule the formal charge on the central oxygen atom is ______.
Options
-1
+2
0
+1
MCQ
Fill in the Blanks
Solution
In ozone molecule the formal charge on the central oxygen atom is +1.
Explanation:
The formal charge can be calculated using formula,
Formal charge = number of valence electrons - `1/2`
(number of electrons in bond pairs) - number of electrons in lone pair.
For O3,
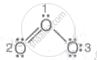
Formal charge for central atom O(1)
`= 6 - 2 - 1/2(6)`
= + 1
shaalaa.com
Oxygen and Compounds of Oxygen
Is there an error in this question or solution?
