Advertisements
Advertisements
Question
In the laboratory preparation of hydrogen from zinc & dilute hydrocholoric acid – state a reason for the collecting the hydrogen by downward displacement of water and not air & collecting it after all the air in the apparatus is allowed to escape.
Solution
Hydrogen is collected by downward displacement of water as it is slightly soluble in water and in air it form explosive mixture with air and also hydrogen is lighter than air.
APPEARS IN
RELATED QUESTIONS
Indicate which of the following statement is true and which is false:
Nitric acid cannot be used to prepare hydrogen by its action on active metals.
Name the following:
A metal which liberates hydrogen only when steam is passed over red hot metal.
FILL IN THE BLANK
........................ zinc is preferred over pure zinc in the laboratory preparation of hydrogen.
Why is granulated zinc preferred in the laboratory preparation of hydrogen?
Give a test to identify hydrogen ?
Give reason for the following:
Copper does not displace hydrogen from dilute hydrochloric acid, but zinc does.
Give a balanced equation for the following conversions sodium plumbite from lead.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The complete apparatus is air-tight.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
Hydrogen is not collected over air.
The diagram represent the preparation and collection of hydrogen by a standard
laboratory method.
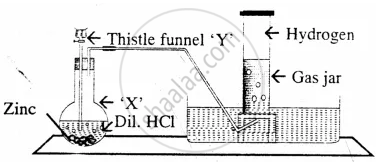
State what is added through the thistle funnel ‘Y’
