Advertisements
Advertisements
Question
Match the items of Column I and Column II.
| Column I | Column II |
| (i) Dialysis | (a) Cleansing action of soap |
| (ii) Peptisation | (b) Coagulation |
| (iii) Emulsification | (c) Colloidal sol formation |
| (iv) Electrophoresis | (d) Purification |
Solution
| Column I | Column II |
| (i) Dialysis | (d) Purification |
| (ii) Peptisation | (c) Colloidal sol formation |
| (iii) Emulsification | (a) Cleansing action of soap |
| (iv) Electrophoresis | (b) Coagulation |
Explanation:
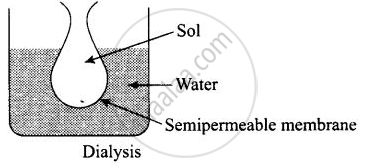
(i) Purification of colloid can be done by dialysis in which ions/particles are removed from solution through semipermeable membrane.
(ii) Peptisation is a process in which when small quantity of electrolyte (peptizing agent) is added to precipitate. It leads to formation of colloidal solution.
(iii) The process of removing of oily or greasy dirt from the cloth is done by emulsification.
(iv) Process of setting of colloidal particle is called coagulation. Electrophoresis is a process in which on applying electric potential to the electrodes dipped in sol, the oppositely charged particles of colloidal solution move towards oppositely charged electrodes, get discharged and precipitated.
APPEARS IN
RELATED QUESTIONS
What type of solutions are formed on dissolving different concentrations of soap in water?
What happens when dialysis is prolonged?
Observe the figure given below and answer the questions that follow:

- Which process is represented in the figure?
- What is the application of this process?
- Can the same process occur without applying electric field? Why is the electric field applied?
Dialysis is the process of separation of ______
