Advertisements
Advertisements
Question
Give the structural formula of Acetic acid.
Solution
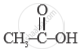
APPEARS IN
RELATED QUESTIONS
A student adds 2 mL of acetic acid to a test tube containing 2 mL of distilled water. He then shakes the test tube well and leaves it to settle for some time. After about 5 minutes he observes that in the test tube there is :
(A) a clear transparent colourless solution
(B) a clear transparent pink solution
(C) a precipitate settling at the bottom of the test tube
(D) a layer of water the layer of acetic acid
Give the common names and IUPAC names of the following compounds of HCOOH.
Name the products formed and give appropriate chemical equations for the following:
sodium reacting with ethyl alcohol.
On adding NaHCO3 to acetic acid, a gas is evolved which turns lime water milky due to the formation of:
(1) Calcium bicarbonate
(2) Calcium hydroxide
(3) Calcium carbonate
(4) Calcium acetate
Name the following:
A toxic alcohol
Write balanced euation for the following :
Ethane is burnt in air.
State the observation
When the gaseous product obtained by dehydration of ethyl alcohol is passed through bromine water.
Observe the figure and write the answers to the following questions.

- Write the name of the reaction shown in the following figure.
- Write the above chemical reaction in the form of a balanced equation.
- Write the name of the product produced in the above reaction, write a use.
- Write the name of the catalyst used in the above reaction.
Bubbles are seen in the test tube during the preparation of lime water.
Why is glacial acetic acid called so?
