Advertisements
Advertisements
Question
Phosphorus has three allotropic forms — (i) white phosphorus (ii) red phosphorus and (iii) black phosphorus. Write the difference between white and red phosphorus on the basis of their structure and reactivity.
Solution
Yellow or white phosphorus: It is a discrete tetrahedral molecule. The molecular formula is P4. The four phosphorus atoms lie at the comers of a regular tetrahedron. Each P atom is linked to each of the three other atoms by single covalent bonds i.e., six P – P bonds are present and each P atom has a lone pair.
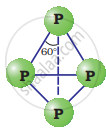
White Phosphorous

Red Phosphorous
APPEARS IN
RELATED QUESTIONS
Out of white phosphorus and red phosphorus, which one is more reactive and why?
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2?
Give reasons for the following:
Red phosphorus is less reactive than white phosphorus.
Which allotrope of sulphur is thermally stable at room temperature ?
Which allotrope of phosphorous is most stable?
All elements of Group 15 show allotropy except ______.
Which of the following elements does not show allotropy?
Which of the following is correct for P4 molecule of white phosphorus?
(i) It has 6 lone pairs of electrons.
(ii) It has six P–P single bonds.
(iii) It has three P–P single bonds.
(iv) It has four lone pairs of electrons.
Which of the following statements are correct?
(i) All the three N – O bond lengths in \[\ce{HNO3}\] are equal.
(ii) All P – Cl bond lengths in \[\ce{PCl5}\] molecule in gaseous state are equal.
(iii) \[\ce{P4}\] molecule in white phosphorus have angular strain therefore white phosphorus is very reactive.
(iv) \[\ce{PCl}\] is ionic in solid state in which cation is tetrahedral and anion is octahedral.
Which one of the following is formed (mainly) when red phosphorus is heated in a sealed tube at 803 K?
