Advertisements
Advertisements
Question
The correct order of increasing the reactivity of C–X bond towards nucleophile in following compounds.
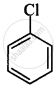
(I)
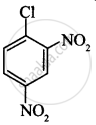
(II)
(CH3)3CCl
(III)
(CH3)2CHCl
(IV)
Options
(IV) < (III) < (I) < (II)
(I) < (II) < (IV) < (III)
(III) < (II) < (I) < (IV)
(II) < (III) < (I) < (IV)
MCQ
Solution
(III) < (II) < (I) < (IV)
Explanation:
(III) is most reactive due to stability of 3° carbocation, −NO2 (electron-withdrawing) group increase nucleophilic substitution reaction.
shaalaa.com
Is there an error in this question or solution?
