Advertisements
Advertisements
Question
The molecule which contains a triple covalent bond is ______.
Options
ammonia
methane
water
nitrogen
Solution
The molecule which contains a triple covalent bond is nitrogen.
APPEARS IN
RELATED QUESTIONS
Name a carbon containing molecule which has two double bonds.
What type of chemical bond is formed between carbon and bromine?
Give the formula of the compound that would be formed by the combination of the following pair of elements:
Al and Cl2
What type of bonding would you expect between Hydrogen and Chlorine?
Why is graphite a good conductor of electricity but diamond is a non-conductor of electricity?
Draw an electron dot structure of the following molecule. (Without showing the circle) :
Methanol
Explain the following term with example.
Alkane
Element A has 2 electrons in its M shell. Element B has atomic number 7.
Write equations to show how A and B form ions.
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
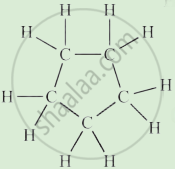 |
Which of the following are correct structural isomers of butane?
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.....}|\phantom{.....}|\\
\ce{H}\ce{H-C-H}\ce{H}\\
|\\
\ce{H}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - H}\\
|\phantom{.....}\backslash\phantom{..}|\\
\phantom{....}\ce{H}\phantom{......}\ce{C - H}\phantom{}\\
\phantom{.......}|\\
\phantom{.......}\ce{H}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H - C - C - H}\\
|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\\
\end{array}\]
