Advertisements
Advertisements
Question
The reaction of C6H5–CH=CH–CH3 with HBr produces:
Options
C6H5CH2CH2CH2Br
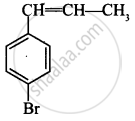
\[\begin{array}{cc}
\ce{C6H5-CH-CH2-CH3}\\
|\phantom{.......}\\
\ce{Br}\phantom{......}
\end{array}\]\[\begin{array}{cc}
\ce{C6H5-CH2-CH-CH3}\\
\phantom{.....}|\\
\phantom{......}\ce{Br}
\end{array}\]
MCQ
Solution
\[\begin{array}{cc}
\ce{C6H5-CH-CH2-CH3}\\
|\phantom{.......}\\
\ce{Br}\phantom{......}
\end{array}\]
Explanation:
Since, its carbocation is stabilised by resonance as well as +I effect.
shaalaa.com
Is there an error in this question or solution?
