Advertisements
Advertisements
Question
The spin only magnetic moment of Mn3+ (Z = 25) in aqueous solution is ____________.
Options
`sqrt3 "BM"`
`2sqrt6 "BM"`
`sqrt6 "BM"`
`3sqrt6 "BM"`
MCQ
Fill in the Blanks
Solution
The spin only magnetic moment of Mn3+ (Z = 25) in aqueous solution is `underline(2sqrt6 "BM")`.
Explanation:
The electronic configuration for Mn3+ (Z = 25) will be
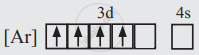
There are 4 unpaired electrons, so n = 4.
∴ µ = `sqrt("n"("n" + 2)) "BM"`
= `sqrt(4(4 + 2))`
= `2sqrt6 "BM"`
shaalaa.com
Trends in Atomic Properties of the First Transition Series
Is there an error in this question or solution?
