Advertisements
Advertisements
Question
The type of mono halogen derivative in which a halogen atom is bonded to sp3 hybridized carbon atom next to carbon-carbon double bond is ______________
Options
alkyl halide
allylic halide
vinylic halide
benzylic halide
Solution
The type of mono halogen derivative in which a halogen atom is bonded to sp3 hybridized carbon atom next to carbon-carbon double bond is allylic halide
APPEARS IN
RELATED QUESTIONS
Butanenitrile may be prepared by heating ______.
Choose the most correct option.
Choose the compound from the following that will react fastest by SN1 mechanism
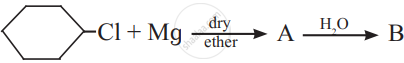
The product ‘B’ in the above reaction sequence is,
Choose the most correct option.
Which of the following is likely to undergo racemization during alkaline hydrolysis?
(I)
\[\begin{array}{cc}
\ce{CH3-CH-C2H5}\\
|\\
\ce{Cl}\end{array}\]
(II)

(III)
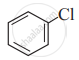
(IV)
\[\begin{array}{cc}
\ce{\phantom{.......}CH3}\\
\phantom{.....}|\\
\ce{CH3-CH}\\
\phantom{.....}|\\
\ce{\phantom{..........}CH2Cl}
\end{array}\]
Match the pairs.
| Column I | Column II |
| \[\begin{array}{cc}\ce{CH3CH - CH3}\\|\phantom{....}\\ \ce{X\phantom{....}}\end{array}\] |
vinyl halide |
| CH2 = CH - CH2X | alkyl halide |
| CH2 = CH - X | allyl halide |
| benzyl halide | |
| aryl halide |
The bond line formula of 1-iodo-2, 3-dimethyl pentane is
When propene reacts with HCl in presence of peroxide, the product is ________.
The conversion of 2-methylpropan-1-ol to 2-methylpropan-2-ol is ______.
IUPAC name of isobutyl chloride is ______.
Alkyl chloride containing 4° carbon atom(s) would be ____________.
Which of the following compounds is obtained when t-butyl bromide is treated with alcoholic ammonia?
The number of possible monohalogen derivatives for the alkyl halide having molecular formula C4H9X is ____________.
How many asymmetric carbon atoms are present in neopentyl chloride?
Identify A and B respectively in the following conversion.
\[\ce{Ethene ->[A] Bromoethane ->[B][\Delta] Ethyl propionate}\]
The decreasing order of the rate of nitration of benzene (I), C6D6 (II), nitrobenzene (III), chlorobenzene (IV) is ______.
The allylic halide, among the following, is ______.
Complete the following reaction giving major product.
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{...................}\\
\ce{CH3 - C - CH2 - Cl ->[Na/dry either] A}\\
|\phantom{...................}\\
\ce{CH3}\phantom{.................}
\end{array}\]
Identify the chiral molecule from the following.
Identify A and B:
\[\ce{C6H5CH2Br ->[Alc.][KCN] A ->[Na/Ethanol] B}\]
Identify the chiral molecule from the following.
Name the following halide according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
\[\ce{CH3C(C2H5)2CH2Br}\]
