Advertisements
Advertisements
Question
Under what conditions can hydrogen be made to combine with chlorine?
Name the products and write the equation for the reaction.
Solution
Equal volumes of hydrogen and chlorine react slowly in diffused sunlight to form hydrogen chloride.
H2 + Cl2 →2HCl
APPEARS IN
RELATED QUESTIONS
Indicate which of the following statement is true and which is false:
Zinc can liberate hydrogen from water, acid and alkali solution.
Give reason for the following:
Nitric acid not used for the preparation of hydrogen gas?
Name the following:
A metallic oxide which can be reduced into metal by hydrogen.
Name the chemicals required to prepare hydrogen gas in the laboratory.
Multiple Choice Question
In metal activity series the more reactive metals are at
FILL IN THE BLANK
Sodium liberates hydrogen when treated with cold .............
FILL IN THE BLANK
........................ zinc is preferred over pure zinc in the laboratory preparation of hydrogen.
Why is granulated zinc preferred in the laboratory preparation of hydrogen?
Starting from zinc how would you obtain hydrogen using a dilute acid.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
Give balanced equation for the following conversion:
Acidified water to hydrogen – by electrolysis.
Give reason for the following:
In the laboratory preparation of hydrogen from zinc and dilute hydrochloric acid – the zinc used granulated zinc.
Give a balanced equation for the following conversions sodium aluminate from aluminium.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
The complete apparatus is air-tight.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
Hydrogen is not collected over air.
Give a reason for the following.
Nitric acid in the dilute form is not used in the laboratory preparation of hydrogen from metals.
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.
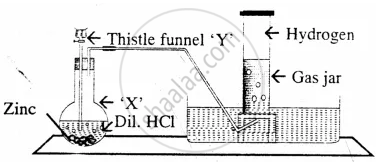
State what difference will be seen if pure zinc is added in the distillation flask ‘X’ instead of granulated zinc.
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

Name a solution which absorbs the impurity – `"H"_2"S"`
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

Name a gas other than hydrogen collected by the same method.
