Advertisements
Advertisements
Question
What is the action of chlorine (Cl) on the following:
Potassium bromide solution
Solution
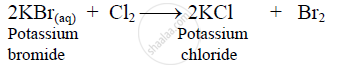
Action of Cl2 on potassium bromide solution:
Chlorine reacts with KBr, to form potassium chloride, displacing bromine.
APPEARS IN
RELATED QUESTIONS
What happens when
(i) PCl5 is heated ? Write the reactions involved.
Give the reason for bleaching action of Cl2.
Name two poisonous gases which can be prepared from chlorine gas.
Write balanced equations for Chlorine gas is passed into a solution of NaI in water.
Arrange the following oxyacids of chlorine – HClO, HClO2, HClO3, and HClO4 with respect to Increasing order of thermal stability.
What is the action of chlorine (Cl) on the following:
Hot and concentrated caustic soda
Arrange the following oxyacids of chlorine – HClO, HClO2, HClO3, and HClO4 with respect to Increasing order of oxidizing power.
Write any two uses of Chlorine.
What is the action of chlorine on CS2
Answer the following.
How is hydrogen chloride prepared from sodium chloride?
What is the action of hydrochloric acid on NH3?
Answer the following.
Give two uses of HCl.
Hot and concentrated alkali NaOH reacts with chlorine to form _______.
When SO2 is passed through an aqueous solution of I2, the solution becomes _______.
What is the action of chlorine on hot and concentrated sodium hydroxide?
Write the chemical reaction of action of Cl2 on excess NH3.
What happens when chlorine reacts with?
Al
What happens when chlorine reacts with?
Na
What happens when chlorine reacts with?
S8
In which the NH3 is not used?
H2S does not produce metallic sulphide with:
When chlorine reacts with a cold and dilute solution of sodium hydroxide, it forms:
The correct order of bond angles in the following species is:
Assertion: \[\ce{NaCl}\] reacts with concentrated \[\ce{H2SO4}\] to give colourless fumes with pungent smell. But on adding \[\ce{MnO2}\] the fumes become greenish yellow.
Reason: \[\ce{MnO2}\] oxidises \[\ce{HCl}\] to chlorine gas which is greenish yellow.
Chlorine water loses its yellow colour on standing because ______.
When chlorine water is exposed to sunlight, the colour change which occurs is form:-
When chlorine is passed over dry slaked lime at room temperature, the product formed is ______
Complete the following reaction:
\[\ce{Na2SO3 + Cl2 + H2O -> \underline{}\underline{}\underline{}\underline{} + \underline{}\underline{}\underline{}\underline{}}\]
Write reactio between Cl2 and water.
Write the chemical reaction when chlorine reacts with dry slaked lime.
What is the action of hydrochloric acid on the NH3?
What is the action of hydrochloric acid on the following NH3?
What is the action of hydrochloric acid on Na2CO3?
What is the action of hydrochloric acid on \[\ce{Na2CO3}\]?
What is the action of hydrochloric acid on NH3?
What is the action of hydrochloric acid on Na2CO3?
What is the action of hydrochloric acid on Na2CO3?
What is the action of hydrochloric acid on \[\ce{NH3}\]?
What is the action of hydrochloric acid on \[\ce{Na2CO3}\]?
What is the action of hydrochloric acid on the following?
\[\ce{NH3}\]
