Advertisements
Advertisements
Questions
What are the types of ligands? Give one example of each type.
Write classification of ligands with one example of each type.
Solution
Depending upon the number of electron donor atoms present, ligands are classified as:
- Monodentate ligands: A monodentate ligand is the one where a single donor atom shares an electron pair to form a coordinate bond with the central metal ion.
e.g. Cl–, OH–, CN–, etc. - Polydentate ligands: A polydentate ligand has two or more donor atoms linked to the central metal ion. Based on the number of donor atoms, polydentate ligands are further classified as:
- Bidentate ligands: The ligands which bind to central metal through two donor atoms are called bidentate ligands.
e.g.- Ethylenediamine binds to the central metal atom through two nitrogen atoms.
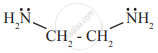
- Similarly, Oxalate ligand `("C"_2"O"_4^{2-})` utilizes electron pair on each of its negatively charged oxygen atoms on linking with central metal.

- Ethylenediamine binds to the central metal atom through two nitrogen atoms.
- Hexadentate ligands: The ligands which bind to central metal through six donor atoms are called hexadentate ligands.
e.g. Ethylenediaminetetraacetate ion (EDTA)4– binds to metal by electron pairs of four oxygen and two nitrogen atoms.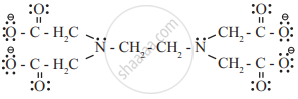
- Bidentate ligands: The ligands which bind to central metal through two donor atoms are called bidentate ligands.
- Ambidentate ligands: The ligands which have two donor atoms and use the electron pair of either donor atoms to form a coordinate bond with the metal ion are called ambidentate ligands.
e.g.- The ligand `"NO"_2^-` links to the metal ion through nitrogen or oxygen.
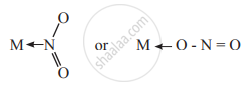
- SCN– has two donor atoms nitrogen and sulphur either of which links to metal M ← SCN– or M ← NCS–.
- The ligand `"NO"_2^-` links to the metal ion through nitrogen or oxygen.
APPEARS IN
RELATED QUESTIONS
What is meant by the chelate effect?
Answer in brief.
What are bidentate ligands? Give one example.
Answer in brief.
What are the coordination number and oxidation state of the metal ion in the complex [Pt(NH3)Cl5]-?
Answer in brief.
What is the difference between a double salt and a complex? Give an example.
Amongst the following, the ambidentate ligand is ____________
SCN− and oxalate ions are examples of ____________ ligands respectively.
Which of the following is an ambidentate ligand?
[EDTA]4− and oxalate ions are examples of ____________ ligands respectively.
Which among the following coordination compounds does not have coordination number equal to number of ligands?
Identify monodentate ligand from following.
What is the number of nitrogen atoms and −COO− groups respectively present in EDTA?
Write a Example monodentate ligand.
What are ligands?
What are ligands types? Give one example of each type.
What are ligands?
What are ligands?
What are ligands?
What are the types of ligands? Give one example of each type.
What are ligands?
What are ligands types? Give one example of each type.
What are the types of ligands? Give one example of each type.
What are the types of ligands? Give one example of each type.
Give an example of chelate effect.
