Advertisements
Advertisements
Question
What do you observe when?
Scrap zinc is heated with conc. HNO3?
Solution
When scrap zinc is heated with conc, HNO3 formation of zinc nitrate, water and nitrogen dioxide will occur.
\[\ce{Zn + 4HNO3 -> Zn(NO3)2 + 2H2O + 2NO2}\]
APPEARS IN
RELATED QUESTIONS
Name the gas evolved when the following mixtures are heated:
Sodium Nitrite and Ammonium Chloride
Give three equations of the reaction to prove that nitric acid is an acid.
Give reason for the following :
Nitric acid usually does not yield hydrogen from acids.
Complete and balance the following equation :
C + HNO3 → ___________
Fill in the blank with appropriate word/words.
98% nitric acid is obtained by distilling 68% nitric acid with ________ under ______
Describe what you see when concentrated nitric acid is added to copper.
The figure given below illustrates the apparatus used in the laboratory preparation of nitric acid: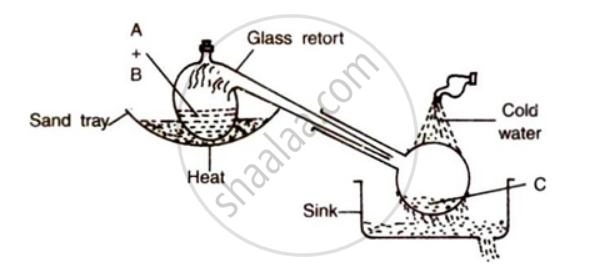
(i) Name A (a liquid), B( a Solid) and C(a liquid).
(ii) write the balanced chemical equation to show how nitric acid undergoes decomposition.
(iii) Write the balanced chemical equation for the reaction in which copper is oxidized by concentrated nitric acid.
Copy and complete the following table relating to important industrial processes output refers to the product of the process not the intermediate steps.
| Name of process | Inputs | Catalyst | Equation for catalysed reaction | Output |
| Haber process | Hydrogen + | |||
|
Ammonia + |
Nitric acid |
Give one test to distinguish between the following pair of chemicals.
Zinc nitrate solution and calcium nitrate solution.
From the following list of substances, choose one substance in the case which matches the description given below:
Ammonium nitrate, calcium hydrogen carbonate, copper carbonate, lead nitrate, potassium nitrate, sodium carbonate, sodium hydrogen carbonate, zinc carbonate.
A Substance that gives off only oxygen when heated.
