Advertisements
Advertisements
Question
What is the difference in the molecular formula of any two adjacent homologues in terms of molecular mass?
Solution
The difference in the molecular formula of any two adjacent homologues is 14 a.m.u.
RELATED QUESTIONS
Give the molecular formula of one homologue of each of the following:
C2H6
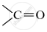 .
.
Give three points to differentiate between saturated and unsaturated
hydrocarbons.
What is the difference in the molecular formula of any two adjacent homologues:
(i) In terms of molecular mass
(ii) In terms of number and kind of atoms per molecule?
The general formula of Alkane is _________________
While going in an increasing order there is a rise in the molecular mass of the consecutive members of the homologous series by _______.
Cyclohexane : Cyclic hydrocarbon : : Isobutylene : _______
Complete the following table for the homologous series of alkanes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methane | CH4 | CH4 | 1 | 1 | -162 |
| Ethane | C2H6 | CH3–CH3 | 2 | 2 | -88.5 |
| Propane | C3H8 | CH3–CH2–CH3 | 3 | 3 | -42 |
| Butane | C4H10 | CH3–CH2–CH2–CH3 | ______ | ______ | 0 |
| Pentane | C5H12 | CH3–CH2–CH2–CH2–CH3 | ______ | ______ | 36 |
| Hexane | C6H14 | CH3–CH2–CH2–CH2–CH2–CH3 | ______ | ______ | 69 |
Write the chemical formula of two consecutive homologous of organic compounds having functional group - OH.
What happens to the (i) boiling point and (ii) solubility of organic compounds of a homologous series as the molecular mass increases?
Name the following:
Group of organic compounds where the successive members follow a regular structural pattern, successive compounds differ by a 'CH2' group.
