Advertisements
Advertisements
Question
What is meant by 'group' in the modern periodic table? How do the following change on moving from top to bottom in a group?
(i) Number of valence electrons
(ii) Number of occupied shells
(iii) Size of atoms
(iv) Metallic character of element
(v) Effective nuclear charge experienced by valence electrons
Solution
Vertical columns of the periodic table are known as groups.
(i) The number of valence electrons remains constant when we move down the group.
(ii) The number of occupied shells increases down the group.
(iii) The size of atoms increases down the group.
(iv) The metallic character of elements increases down the group.
(v) The effective nuclear charge decreases down the group.
APPEARS IN
RELATED QUESTIONS
Two elements ‘A’ and ‘B’ belong to the 3rd period of Modern periodic table and are in group 2 and 13, respectively. Compare their following characteristics in tabular form:-
(a) Number of electrons in their atoms
(b) Size of their atoms
(c) Their tendencies to lose electrons
(d) The formula of their oxides
(e) Their metallic character
(f) The formula of their chlorides
Rewrite the following statement after correction, if necessary:
Elements in the same period have equal valency
An element X belongs to group 2 and another element Y belongs to group 15 of the periodic table:
(a) What is the number of valence electron in X?
(b) What is the valency of X?
(c) What is the number of valence electrons in Y?
(d) What is the valency of Y?
Explain how you have arrived at your answers.
Where would you locate the element with electronic configuration 2, 8 in the modern periodic table?
Periodicity is observed due to the similar ______.
An element A has atomic number 14. To which period does this element belong and how many elements are there in this period.
An element X belong to 4th period and 17th group, state.
name of the element.
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 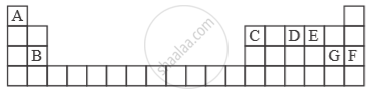
Which is an inert gas?
Fill in the blank
There are ______ groups and ______ periods in the modern form of periodic table.
The elements of one short period of the periodic table are given below in order from left to right:
| Li | Be | B | C | O | F | Ne |
To which period do these elements belong?
