Advertisements
Advertisements
Question
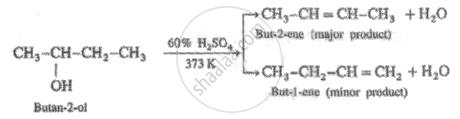
When Butan-2-ol is dehydrated using sulphuric acid, the concentration of acid and temperature needed respectively is _______.
Options
95% cone. and 373 K
60% cone. and 373 K
20% cone. and 363 K
20% cone. and 373 K
MCQ
Fill in the Blanks
Solution
When Butan-2-ol is dehydrated using sulphuric acid, the concentration of acid and temperature needed respectively is 60% cone. and 373 K.
Explanation:
Secondary alcohol (2°) is dehydrated by heating with 60% H2SO4 at 373 K.
shaalaa.com
Alkanes
Is there an error in this question or solution?
