Advertisements
Advertisements
Question
Which among the following crystal lattices occupies all of the cubic holes by cations?
Options
UO2
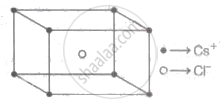
CsCI
CaF2
SrCl2
MCQ
Solution
CsCI
Explanation:
Cations inhabited all of the cubic holes in CsCI crystal lattices. A single atom in the cube's centre belongs to this unit cell, and the unit cell has only one body centre. As a result, the compound's formula is CsCI, which stands for body-centred cubic cell. Particles are present in the corners of the cube, as well as one particle in the centre of the body, in this sort of cell.
shaalaa.com
Crystal Structure
Is there an error in this question or solution?
