Advertisements
Advertisements
Question
Which among the following pairs of elements in their respective oxidation states will have same value of effective magnetic moment?
(Atomic number: Sc = 21, Ti = 22, Cr = 24, Co = 27, Ni = 28, Zn = 30)
Options
Zn2+ and Cr3+
Ni2+ and Ti3+
Sc3+ and Ti3+
Cr3+ and CO2+
MCQ
Solution
Cr3+ and CO2+
Explanation:
| Configuration | No. of unpaired electrons | |
| Sc3+ = 4s0 3d0 |  |
0 |
| Cr3+ = 4s0 3d3 | 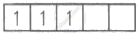 |
3 |
| Ti3+ = 4s0 3d1 | 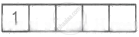 |
1 |
| Ni2+ = 4s0 3d8 | 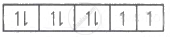 |
2 |
| Co2+ = 4s0 3d7 | 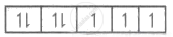 |
3 |
Species having same number of unpaired electrons have same dipole moment:
Cr3+ & CO2+ have 3 unpaired electrons.
`mu = sqrt("n"("n + 2")) = sqrt15` = 3.9
shaalaa.com
Trends in Atomic Properties of the First Transition Series
Is there an error in this question or solution?
