Advertisements
Advertisements
Question
Which of the following compounds are aromatic according to Huckel’s rule?
 |
 |
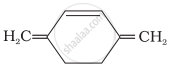 |
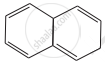
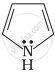
| (A) | (B) | (C) |
 |
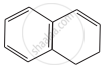 |
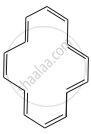 |
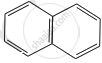
| (D) | (E) | (F) |
Solution
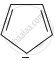
A = Has 8π electrons, does not follow Huckel rule. Orbitals of one carbon atom are not in conjugation. It is not aromatic.
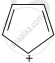
B = Has 6π delocalised electrons. Hence, is aromatic.
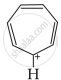
C = Has 6π electrons in conjugation but not in the ring. Non-aromatic.
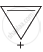
D = 10π electrons in planar rings, aromatic.
E = Out of 8π electrons it has delocalised 6π electrons in one six-membered planar ring, which follows Huckel’s rule due to which it will be aromatic.
F = 14π electrons are in conjugation and are present in a ring. Huckel’s rule is being followed. Compound will be aromatic if ring is planar.
APPEARS IN
RELATED QUESTIONS
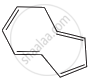
The ring systems having following characteristics are aromatic.
(i) Planar ring containing conjugated π bonds.
(ii) Complete delocalisation of the π−electrons in ring system i.e. each atom in the ring has unhybridised p-orbital, and
(iii) Presence of (4n + 2) π−electrons in the ring where n is an integer (n = 0, 1, 2,...........) [Huckel rule].
Using this information classify the following compounds as aromatic/non-aromatic.
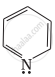 |
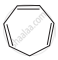 |
 |
 |
| (A) | (B) | (C) | (D) |
 |
 |
 |
|
| (E) | (F) | (G) |
\[\ce{CH ≡ CH ->[NH4Cl][Cu2Cl2] Product}\]
Product is:
When 2-alkyne is treated with sodamide product will be ______.
