Advertisements
Advertisements
Question
Which of the following compounds would undergo SN1 reaction faster and why?
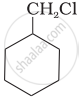 |
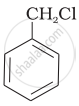 |
| (A) | (B) |
Short Note
Solution
(B) Undergoes SN1 reaction faster than (A) because in the case of (B), the carbocation formed after the loss of Cl– is stabilised by resonance.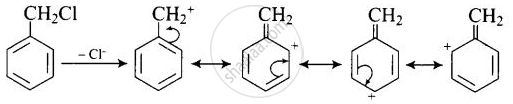
On the other hand, the carbonation formed during reaction (A) is not resonance stabilized.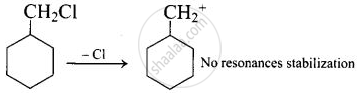
shaalaa.com
Is there an error in this question or solution?
Chapter 10: Haloalkanes and Haloarenes - Exercises [Page 144]
