Advertisements
Advertisements
Question
Which of the following is likely to undergo racemisation during alkaline hydrolysis?
Options
\[\ce{(CH3)3C - CH2 - Cl}\]
\[\begin{array}{cc}
\ce{H3C - CH - CH3}\\
|\phantom{..}\\
\ce{Cl}\phantom{.}
\end{array}\]\[\begin{array}{cc}
\ce{H3C - CH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{......}\ce{Cl}
\end{array}\]\[\begin{array}{cc}
\ce{H3C - CH2 - CH - CH2 - CH3}\\
|\phantom{..}\\
\ce{Cl}\phantom{.}
\end{array}\]
MCQ
Solution
\[\begin{array}{cc}
\ce{H3C - CH2 - CH - CH3}\\
\phantom{.....}|\\
\phantom{......}\ce{Cl}
\end{array}\]
Explanation:
Among the given compounds,
\[\begin{array}{cc}\ce{H3C - CH2 - CH - CH3}\\
\phantom{.....}|\\\phantom{......}\ce{Cl}\end{array}\] will undergo racemisation during alkaline hydrolysis. The reaction involved is as follows:
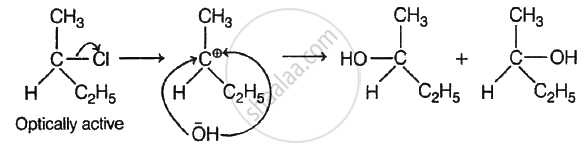
shaalaa.com
Alkali Metals and Alkaline Earth Metals
Is there an error in this question or solution?
