Advertisements
Advertisements
Question
Which of the following is the most stable carbocation?
Options
\[\ce{\overset{⊕}{C}H3}\]
\[\ce{CH3-\overset{⊕}{C}H2}\]
\[\begin{array}{cc}
\phantom{........}\ce{O}\\
\phantom{........}||\\
\ce{CH3 - \underset{⊕}{C}}
\end{array}\]\[\begin{array}{cc}
\ce{CH3}\phantom{.......}\\
\backslash\phantom{...}\\
\ce{CH3 - C - \overset{⊕}{C}H2}\\
/\phantom{...}\\
\ce{CH3}\phantom{.......}
\end{array}\]
MCQ
Solution
\[\begin{array}{cc}
\phantom{........}\ce{O}\\
\phantom{........}||\\
\ce{CH3 - \underset{⊕}{C}}
\end{array}\]
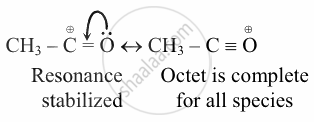
Explanation:
The carbocation that is bound to a hetero atom with a single electron pair is more than just a homoatomic attachment.
shaalaa.com
Fundamental Concepts in Organic Reaction Mechanism - Fission of a Covalent Bond
Is there an error in this question or solution?
