Advertisements
Advertisements
Question
Which of the following reactions belong to electrophilic aromatic substitution?
(i) Bromination of acetanilide
(ii) Coupling reaction of aryldiazonium salts
(iii) Diazotisation of aniline
(iv) Acylation of aniline
Solution
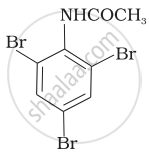
(i) Bromination of acetanilide
(ii) Coupling reaction of aryldiazonium salts
Explanation:
Benzene diazonium chloride reacts with phenol to form p-hydroxy azobenzene by coupling the phenol molecule at its para-position with the diazonium salt. This is referred to as a coupling reaction. This is an illustration of an electrophilic substitution reaction.

At room temperature, aniline reacts with bromine water to form a white precipitate of 2, 4, 6-tribromoaniline.
By acetylation with acetic anhydride to protect the –NH2 group, then carrying out the desired substitution followed by hydrolysis of the substituted amide to the substituted amine.

APPEARS IN
RELATED QUESTIONS
Write short notes on the following Coupling reaction
Illustrate the following reactions giving suitable example in each case
Coupling reaction
Write equations of the following reactions:
Coupling reaction
Which of the following compound will not undergo azo coupling reaction with benzene diazonium chloride?
The product of the following reaction is:
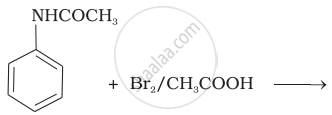
(i)
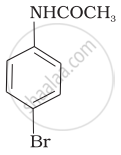
(ii)
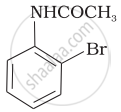
(iii)
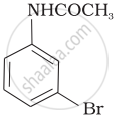
(iv)
Under what reaction conditions (acidic/basic), the coupling reaction of aryldiazonium chloride with aniline is carried out?
Benzenediazonium chloride reacts with phenol to give p-hydroxy azobenzene, an orange dye. This reaction is known as ______.
When benzene diazonium chloride reacts with phenol, it forms a dye. This reaction is called ______.
