Advertisements
Advertisements
Question
Write a note on Clemmensen reduction.
Answer in Brief
Solution
- The carbonyl group
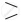 C = O on reduction with zinc amalgam (Zn–Hg ) in concentrated hydrochloric acid is converted into methylene group (–CH2 –).
C = O on reduction with zinc amalgam (Zn–Hg ) in concentrated hydrochloric acid is converted into methylene group (–CH2 –).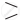 \[\ce{C = O + 4[H] ->[Zn–Hg][conc. HCl] = CH2- + H2O}\]
\[\ce{C = O + 4[H] ->[Zn–Hg][conc. HCl] = CH2- + H2O}\] - Aldehydes and ketones on reaction with Zn –Hg in concentrated HCl forms corresponding alkanes. This reduction is called Clemmensen reduction.
\[\begin{array}{cc}
\ce{O}\phantom{.............................}\\
||\phantom{.............................}\\
\ce{\underset{Ketone}{R-C}-R + 4[H] ->[Zn –Hg][conc. HCl] \underset{Alkane}{R–CH2 –R}}
\end{array}\] - Acetaldehyde on reduction with Zn–Hg in concentrated HCl forms ethane.
\[\begin{array}{cc}
\ce{O}\phantom{...................}\\
//\phantom{.....................}\\
\ce{CH3 - C + 4 [H] ->[Zn–Hg][conc. HCl] \underset{Ethane}{C2H6} + H2O}\\
\backslash\phantom{.....................}\\
\ce{\underset{Acetaldehyde}{H}}\phantom{....................}
\end{array}\]
shaalaa.com
Chemical Properties of Aldehydes and Ketones
Is there an error in this question or solution?
