Advertisements
Advertisements
Question
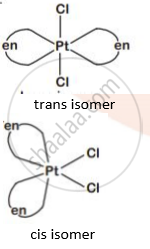
Write IUPAC name of the complex [Pt(en)2Cl2]. Draw structures of geometrical isomers for this complex.
Solution
`["Pt"("en")_2 "Cl"_2]` - Dichloridobis (ethane-1,2,-diammine) platinum(II)
Geometrical isomer:
APPEARS IN
RELATED QUESTIONS
The complex ion `[Co(H_2O)_5 (ONO)]^(2+) `
(A) linkage isomer
(B) ionisation isomer
(C) co-ordination isomer
(D) geometrical isomer
Give evidence that [Co(NH3)5Cl]SO4 and [Co(NH3)5SO4]Cl are ionization isomers.
What type of isomerism is shown by the complex [Co(en)3]Cl3
Write the IUPAC name of the complex [Cr(NH3)4Cl2]+. What type of isomerism does it exhibit?
Which of the following isomer has the highest melting point?
The compounds \[\ce{[CO(SO4)(NH3)5]Br}\] and \[\ce{[Co(SO4)(NH3)5]Cl}\] represent
What kind of isomerism exists between \[\ce{[Cr(H2O)6]Cl3}\] (violet) and \[\ce{[Cr(H2O)5Cl]Cl2 . H2O}\] (greyish-green)?
Assertion: Linkage isomerism arises in coordination compounds containing ambidentate ligand.
Reason: Ambidentate ligand has two different donor atoms
CoSO4Cl.5NH3 exists in two isomeric forms ‘A’ and ‘B’. Isomer ‘A’ reacts with AgNO3 to give white precipitate, but does not react with BaCl2. Isomer ‘B’ gives white precipitate with BaCl2 but does not react with AgNO3. Answer the following questions.
- Identify ‘A’ and ‘B’ and write their structural formulas.
- Name the type of isomerism involved.
- Give the IUPAC name of ‘A’ and ‘B’.
Which isomer of C5H10 gives a single monochloro compound C5H9Cl in bright sunlight?
