Advertisements
Advertisements
Question
Write the mechanism of free radical polymerization of ethene.
Solution
Free radical mechanism for polymerisation of ethene
Chain-initiating step
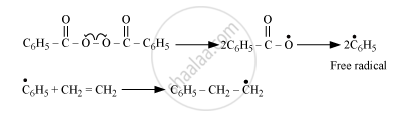
Chain-propagating step
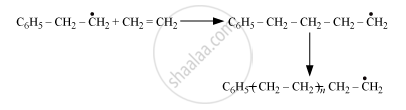
Chain-terminating step

APPEARS IN
RELATED QUESTIONS
Which of the following is a natural polymer ? Buna-S, Proteins, PVC
Write the free radical mechanism for the polymerisation of ethene.
Which of the following statements is not true about low-density polythene?
Match the polymer of column I with correct monomer of column II.
| Column I | Column II |
| (i) High density polythene | (a) Isoprene |
| (ii) Neoprene | (b) Tetrafluoroethene |
| (iii) Natural rubber | (c) Chloroprene |
| (iv) Teflon | (d) Acrylonitrile |
| (v) Acrilan | (e) Ethene |
Match the polymers given in Column I with their chemical names given in Column II.
| Column I | Column II |
| (i) Nylon 6 | (a) Polyvinyl chloride |
| (ii) PVC | (b) Polyacrylonitrile |
| (iii) Acrilan | (c) Polycaprolactum |
| (iv) Natural rubber | (d) Low-density polythene |
| (v) LDP | (e) cis-polyisoprene |
Match the polymers given in Column I with the preferred mode of polymerisation followed by their monomers.
| Column I | Column II |
| (i) Nylon-6,6 | (a) Free radical polymerisation |
| (ii) PVC | (b) Ziegler-Natta polymerisation or coordination polymerisation |
| (iii) HDP | (c) Anionic polymerisation |
| (d) Condensation polymerisation |
The Ziegler Natta catalyst are
Which one of the following polymers are prepared by addition polymerisation?
Orlon fibres are made up of ______.
\[\ce{X + C + Cl2 ->[High temperature][of about 1000 K] Y + CO}\];
\[\ce{Y + 2H2O -> Z + 2HCl}\]
Compound Y is found in polymeric chain structure and is an electron-deficient Molecule. Y must be:
