Advertisements
Advertisements
Question
Write the molecular formula of first two members of homologous series having functional group – OH
Solution
The general formula for the homologous series of functional group −OH is CnH2n+1OH.
The two consecutive members of this series are:
a. CH3OH(Methanol)
b. CH3CH2OH (Ethanol)
where n is 1 and 2 respectively
The molecular formula for both of them are CH4O and C2H6O.
APPEARS IN
RELATED QUESTIONS
Write the molecular formula of first two members of homologous series having functional group -Cl.
The molecular formula of a homologue of butane is:
(a) C4H8
(b) C3H6
(c) C4H6
(d) C3H8
The molecular formula of the third member of the homologous series of ketones is:
(a) C4H8O
(b) C3H6O
(c) C5H10O
(d) C6H12O
Give the structure of the second member of the alcohol group
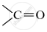 .
.
Find the odd one out and give its explanation.
Complete the following table for the homologous series of alkanes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methane | CH4 | CH4 | 1 | 1 | -162 |
| Ethane | C2H6 | CH3–CH3 | 2 | 2 | -88.5 |
| Propane | C3H8 | CH3–CH2–CH3 | 3 | 3 | -42 |
| Butane | C4H10 | CH3–CH2–CH2–CH3 | ______ | ______ | 0 |
| Pentane | C5H12 | CH3–CH2–CH2–CH2–CH3 | ______ | ______ | 36 |
| Hexane | C6H14 | CH3–CH2–CH2–CH2–CH2–CH3 | ______ | ______ | 69 |
C3H8 belongs to the homologous series of ______.
Consider the carbon compounds having following molecular formula:
(i) C3H6 (ii) C3H8 (iii) C4H6 (iv) C6H6 (v) C6H12
- State the number of double covalent bonds present in C3H6.
- Write the formula of first member of the homologous series to which the carbon compound C4H6 belongs.
- Which one of the above compounds forms a ring structure of carbon atoms?
- Identify, which of the above compounds, is a member of alkane series.
Consider the carbon compounds having following molecular formula:
(i) C2H2 (ii) C2H5 (iii) C3H7OH (iv) C2H6COOH (v) CH3CHO
- Identify which one of the above compounds, is a member of aldehyde series.
- Write the general formula of the series to which compound C2H2 belongs.
- Which one of the above compounds has triple bonds between carbon-carbon atoms?
- Write the molecular formula of the first member of the homologous series to which the compound C3H7OH belongs.
