Advertisements
Advertisements
Question
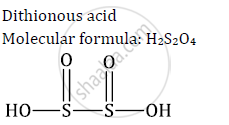
Write molecular formulae and structures of the Dithionous acid
Solution
APPEARS IN
RELATED QUESTIONS
The molecular formula H2S2O2 represents which oxoacid?
- Hydrosulphurous acid
- Thiosulphurous acid
- Sulphuric acid
- Pyrosulphurous acid
Write molecular formulae and structures of Dithionic acid
Write molecular formulae and structures of the Peroxy monosulphuric acid
Write molecular formula and structure of Pyrosulphuric acid
Write the structures of the following molecules: H2SO3
Draw the structures of the following molecules: H2S2O8
What happens when sulphur dioxide is passed through an aqueous solution of Fe(III) salt?
Write the molecular and structural formulae of Dithionous acid
Account for the following :
H3PO2 is a stronger reducing agent than H3PO3.
Write one difference between transition elements and p-block elements with reference to variability of oxidation states.
In which of the pair of ions, both species contain S–S bond?
Which of the following are peroxoacids of sulphur?
An amorphous solid “A” burns in air to form a gas “B” which turns lime water milky. The gas is also produced as a by-product during roasting of sulphide ore. This gas decolourises acidified aqueous \[\ce{KMnO4}\] solution and reduces \[\ce{Fe^{3+}}\] to \[\ce{Fe^{2+}}\]. Identify the solid “A” and the gas “B” and write the reactions involved.
Bromine water react with SO2 to from.
Write uses of sulphur.
Which of the following oxoacids of sulphur contains "S" in two different oxidation states?
