Advertisements
Advertisements
Question
Write the names and the formula of the products formed when
methane Reacts with chlorine Write the chemical equations
Solution
When methane reacts with chlorine in the presence of sunlight or UV light, it undergoes substitution reaction to form Tetrachloromethane.
`CH_4+Cl_2`  `CH_3Cl+HCl`
`CH_3Cl+HCl`
Chloromethane
`CH_3CL+CL_2` 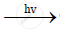 `CH_2CL_2+HCL`
`CH_2CL_2+HCL`
Dichloromethane
`CH_2CL_2+CL_2` 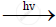 `CHCL_3+HCI`
`CHCL_3+HCI`
Trichloromethane
`CHCL_3+CL_2` 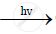 ` C CL_4+HCL`
` C CL_4+HCL`
Tetrachloromethane
shaalaa.com
Hydrocarbons: Alkanes
Is there an error in this question or solution?
