Advertisements
Advertisements
Question
Write the equation for the preparation of carbon tetrachloride from methane.
Solution 1
\[\ce{CH4 + 4Cl2 ->[CuCl][\Delta] CCl4 + 4HCl}\]
Solution 2
\[\ce{CH4 + Cl2 ->[Diffused sunlight][or 600K] CH3Cl + HCl}\]
\[\ce{CH3Cl + Cl2 -> CH2Cl2 + HCl}\]
\[\ce{CH2Cl2 + Cl2 ->CHCl3 + HCl}\]
\[\ce{CHCl3 + Cl2 ->CCl4 + HCl}\]
RELATED QUESTIONS
The equation for the reaction when compound A is bubbled through bromine dissolved in carbon tetrachloride is as follows:
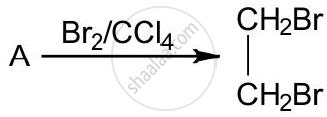
1) Draw the structure of A.
2) State your observation during this reaction.
Write any four properties of organic compounds that distinguish them from inorganic compounds.
Define a functional group with two examples. write the Functional groups for
(i) Alcohol
(ii) Ketone
(iii) Carboxylic acid
Give three uses of ethene.
(a) Draw the structural formula of ethene.
(b) What is the feature of the ethene structure, which allows ethene to react with chlorine in the way it does?
Which compound should be heated with soda lime to obtain ethane gas in the laboratory?
Compound A is bubbled through bromine dissolved in carbon tetrachloride and the product is CH2Br - CH2Br.
\[\ce{A ->[Br2/Od4] CH2Br - CH2Br}\]
What type of reaction has A undergone?
Give balanced equation for the laboratory preparations of the following organic compound:
A saturated hydrocarbon from iodomethene
Give a chemical test to distinguish between Saturated and Unsaturated?
Give the IUPAC name of the organic compound represented by the structural formula given below:
\[\begin{array}{cc}
\ce{H}\phantom{...}\ce{Cl}\phantom{..}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H - C - C - C - C - C - H}\\
|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{Cl}\phantom{..}\ce{H}\phantom{...}\ce{H}\end{array}\]
