Advertisements
Advertisements
Question
Yellow light emitted from a sodium lamp has a wavelength (λ) of 580 nm. Calculate the frequency (ν) and wave number (
Solution
From the expression,
We get,
Where,
ν = frequency of yellow light
c = velocity of light in vacuum = 3 × 108 m/s
λ = wavelength of yellow light = 580 nm = 580 × 10–9 m
Substituting the values in expression (i):
Thus, frequency of yellow light emitted from the sodium lamp
= 5.17 × 1014 s–1
Wave number of yellow light,
APPEARS IN
RELATED QUESTIONS
Find energy of each of the photons which have the wavelength of 0.50 Å.
A photon of wavelength 4 × 10–7 m strikes on the metal surface, the work function of the metal being 2.13 eV. Calculate
- the energy of the photon (eV),
- the kinetic energy of the emission, and
- the velocity of the photoelectron (1 eV = 1.6020 × 10–19 J).
A 25-watt bulb emits monochromatic yellow light of the wavelength of 0.57μm. Calculate the rate of emission of quanta per second.
Following results are observed when sodium metal is irradiated with different wavelengths. Calculate (a) threshold wavelength and, (b) Planck’s constant.
| λ (nm) | 500 | 450 | 400 |
| v × 10-5 (cm s-1) | 2.55 | 4.35 | 5.35 |
In astronomical observations, signals observed from the distant stars are generally weak. If the photon detector receives a total of 3.15 × 10–18 J from the radiations of 600 nm, calculate the number of photons received by the detector.
The work function for caesium atom is 1.9 eV. Calculate
- the threshold wavelength and
- the threshold frequency of the radiation. If the caesium element is irradiated with a wavelength 500 nm, calculate the kinetic energy and the velocity of the ejected photoelectron.
The ejection of the photoelectron from the silver metal in the photoelectric effect experiment can be stopped by applying the voltage of 0.35 V when the radiation 256.7 nm is used. Calculate the work function for silver metal.
If travelling at same speeds, which of the following matter waves have the shortest wavelength?
Which of the following will not show deflection from the path on passing through an electric field?
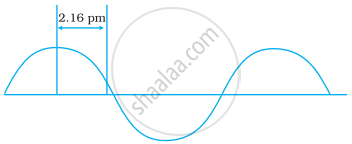
A hypothetical electromagnetic wave is shown in Figure. Find out the wavelength of the radiation.
What is photoelectric effect? State the result of photoelectric effect experiment that could not be explained on the basis of laws of classical physics. Explain this effect on the basis of quantum theory of electromagnetic radiations.
