Topics
Solid State
- General Characteristics of Solid State
- Amorphous and Crystalline Solids
- Classification of Crystalline Solids
- Crystal Lattices and Unit Cells
- Crystal Lattices and Unit Cells - Primitive and Centred Unit Cells
- Number of Atoms in a Unit Cell
- Close Packed Structures of Solids
- Close Packed Structures - Formula of a Compound and Number of Voids Filled
- Packing Efficiency in hcp and ccp Structures
- Efficiency of Packing in Body-centred Cubic Structures
- Packing Efficiency in Simple Cubic Lattice
- Calculations Involving Unit Cell Dimensions
- Imperfections in Solids - Introduction
- Types of Point Defects - Stoichiometric Defects
- Types of Point Defects - Impurity Defects
- Types of Point Defects - Non-stoichiometric Defects
- Electrical Properties - Introduction
- Conduction of Electricity in Metals
- Conduction of Electricity in Semiconductors
- Applications of n-type and p-type Semiconductors
- Magnetic Properties
- Band Theory of Metals
- Solid State Numericals
Solutions
- Introduction of Solution
- Types of Solutions
- Expressing Concentration of Solutions
- Introduction of Solubility
- Solubility of a Solid in a Liquid
- Solubility of a Gas in a Liquid
- Vapour Pressure of Liquid Solutions - Introduction
- Vapour Pressure of Liquid- Liquid Solutions
- Raoult’s Law as a Special Case of Henry’s Law
- Vapour Pressure of Solutions of Solids in Liquids
- Ideal and Non-ideal Solutions
- Colligative Properties and Determination of Molar Mass - Introduction
- Relative Lowering of Vapour Pressure
- Elevation of Boiling Point
- Depression of Freezing Point
- Osmosis and Osmotic Pressure
- Reverse Osmosis and Water Purification
- Abnormal Molar Masses
- Solution Numericals
- Quantitative Concentration Numericals
- Composition of Vapour Phase
- Kohlrausch's law
Electrochemistry
- Introduction to Electrochemistry
- Electrochemical Cells
- Galvanic or Voltaic Cells - Introduction
- Galvanic Cells - Measurement of Electrode Potential
- Nernst Equation - Introduction
- Equilibrium Constant from Nernst Equation
- Electrochemical Cell and Gibbs Energy of the Reaction
- Conductance of Electrolytic Solutions - Introduction
- Measurement of the Conductivity of Ionic Solutions
- Variation of Conductivity and Molar Conductivity with Concentration
- Electrolytic Cells and Electrolysis - Introduction
- Products of Electrolysis
- Primary Batteries
- Secondary Batteries
- Fuel Cells
- Corrosion of Metals
- Relation Between Gibbs Energy Change and Emf of a Cell
- Lead Accumulator
- Faraday’s Law of Induction
Chemical Kinetics
- Rate of Chemical Reaction
- Factors Influencing Rate of a Reaction
- Integrated Rate Equations
- Zero Order Reactions
- First Order Reactions
- Half Life Period of a Reaction
- Pseudo First Order Reaction
- Temperature Dependence of the Rate of a Reaction
- Collision Theory of Chemical Reactions
- Effect of Catalyst on the Rate of Reaction
- Kinetic Energy of Molecule
- Role of Catalyst
- Rate Law and Specific Rate Constant
d-block and f-block Elements
- General Introduction of "D" and "F" Block Element
- Position in the Periodic Table - d-block Elements
- Electronic Configurations of the D-block Elements
- General Properties of the Transition Elements (D-block)
- Some Important Compounds of Transition Elements - Oxides and Oxoanions of Metals
- The Lanthanoids
- The Actinoids
- Some Applications of d and f Block Elements
- "D" and "F" Block Elements Numericals
Surface Chemistry
- Introduction of Adsorption
- Distinction Between Adsorption and Absorption
- Mechanism of Adsorption
- Types of Adsorption
- Adsorption Isotherms (Freundlich and Langmuir Adsorption Isotherm)
- Adsorption from Solution Phase
- Applications of Adsorption
- Homogeneous and Heterogeneous Catalysis
- Adsorption Theory of Heterogeneous Catalysis
- Shape-selective Catalysis by Zeolites
- Enzyme Catalysis
- Catalysts in Industry
- Colloids
- Classification Based on Physical State of Dispersed Phase and Dispersion Medium
- Classification Based on Nature of Interaction Between Dispersed Phase and Dispersion Medium
- Classification Based on Type of Particles of the Dispersed Phase, Multimolecular, Macromolecular and Associated Colloids
- Preparation of Colloids
- Purification of Colloidal Solutions
- Properties of Colloidal Solutions
- Emulsions
- Colloids Around Us
Coordination Compounds
- Introduction of Coordination Compounds
- Werner’s Theory of Coordination Compounds
- Definitions of Some Important Terms Pertaining to Coordination Compounds
- Types of Ligands
- Nomenclature of Coordination Compounds - Formulas of Mononuclear Coordination Entities
- Nomenclature of Coordination Compounds - Naming of Mononuclear Coordination Compounds
- Isomerism in Coordination Compounds
- Stereoisomerism
- Structural Isomerism
- Bonding in Coordination Compounds - Introduction
- Valence Bond Theory (VBT)
- Magnetic Properties of Coordination Compounds
- Crystal Field Theory (CFT)
- Colour in Coordination Compounds
- Bonding in Metal Carbonyls
- Stability of Coordination Compounds
- Importance and Applications of Coordination Compounds
- Coordination Compounds Numerical
General Principles and Processes of Isolation of Elements
- Occurrence of Metals
- Types of Separation or Concentration of an Ore
- Hydraulic Washing
- Magnetic Separation
- Froth Floatation Method
- Leaching
- Extraction of Crude Metal from Concentrated Ore
- Thermodynamic Principles of Metallurgy
- Application of Thermodynamic Principles of Metallurgy
- Electrochemical Principles of Metallurgy
- Oxidation Reduction
- Refining of Crude Metals
- Principles and Methods of Extraction - Concentration
- Uses of Aluminium, Copper, Zinc and Iron
- General Principles and Processes of Isolation of Elements Numerical
Haloalkanes and Haloarenes
- Introduction of Haloalkanes and Haloarenes
- Classification of Haloalkanes and Haloarenes
- Nomenclature
- Nature of C-X Bond
- Methods of Preparation of Haloalkanes
- Methods of Preparation of Haloarenes
- Physical Properties of Haloalkanes and Haloarenes
- Reactions of Haloalkanes - Nucleophilic Substitution Reactions
- Reactions of Haloalkanes - Elimination Reactions
- Reactions of Haloalkanes - Reaction with Metals
- Reactions of Haloarenes - Nucleophilic Substitution
- Reactions of Haloarenes - Electrophilic Substitution Reactions
- Reactions of Haloarenes - Reaction with Metals
- Polyhalogen Compounds
- R-s and D-l Configuration
- Haloalkanes and Haloarenes Numericals
P - Block Elements
- Concept of Group 15 Elements
- Dinitrogen
- Ammonia
- Oxides of Nitrogen
- Nitric Acid
- Phosphorus - Allotropic Forms
- Compounds of Phosphorus
- Phosphine
- Phosphorus Halides
- Oxoacids of Phosphorus
- Concept of Group 16 Elements
- Dioxygen
- Classification of Oxides
- Simple Oxides
- Ozone
- Sulphur - Allotropic Forms
- Compounds of Sulphur
- Sulphur Dioxide
- Oxoacids of Sulphur
- Sulphuric Acid
- Concept of Group 17 Elements
- Compounds of Halogens
- Chlorine
- Hydrogen Chloride
- Oxoacids of Halogens
- Interhalogen Compounds
- Concept of Group 18 Elements
- P Block Elements
Alcohols, Phenols and Ethers
- Classification of Alcohols and Phenols
- Classification of Ethers
- Nomenclature
- Structures of Functional Groups of Alcohols, Phenols and Ethers
- Methods of Preparation of Alcohols
- Methods of Preparation of Phenols
- Physical and Chemical Properties of Alcohols and Phenols
- Reactions Involving Cleavage of O-H Bond
- Reactions Involving Cleavage of Carbon–Oxygen (C–O) Bond in Alcohols
- Chemical Properties of Phenol
- Preparation of Commercially Important Alcohols
- Preparation of Ethers
- Physical Properties of Ethers
- Chemical Reaction of Ethers - Cleavege of C-O Bonds
- Chemical Reaction of Ethers - Electrophilic Substitution
Aldehydes, Ketones and Carboxylic Acids
- Introduction of Aldehydes, Ketones and Carboxylic Acids
- Nomenclature of Aldehydes and Ketones
- Nature of Carbonyl Group
- Structure of the Carbonyl Group
- Preparation of Aldehydes and Ketones
- Preparation of Aldehydes
- Preparation of Ketones
- Physical Properties of Aldehydes and Ketones
- Chemical Reactions of Aldehydes and Ketones - Nucleophilic Addition Reactions
- Chemical Reactions of Aldehydes and Ketones - Reduction
- Chemical Reactions of Aldehydes and Ketones - Oxidation
- Chemical Reactions of Aldehydes and Ketones - Reactions Due to α-hydrogen
- Chemical Reactions of Aldehydes and Ketones - Other Reactions
- Uses of Aldehydes and Ketones
- Introduction of Carboxylic Acids
- Nomenclature of Carboxylic Acids
- Structure of the Carboxyl group
- Methods of Preparation of Carboxylic Acids
- Physical Properties of Carboxylic Acids
- Chemical Reactions of Carboxylic Acids - Reactions Involving Cleavege of O-H Bond
- Chemical Reactions of Carboxylic Acids - Reactions Involving Cleavege of C-OH Bond
- Chemical Reactions of Carboxylic Acids - Reactions Involving –COOH Group
- Chemical Reactions of Carboxylic Acids - Substitution Reactions in the Hydrocarbon Part
- Uses of Carboxylic Acids
Amines
- Introduction of Amines
- Structure of Amines
- Classification of Amines
- Nomenclature of Animes
- Preparation of Amines
- Physical Properties of Amines
- Chemical Reactions of Amines - Basic Character of Amines
- Chemical Reactions of Amines - Alkylation and Acylation
- Chemical Reactions of Amines - Carbylamine Reaction
- Chemical Reactions of Amines - Reaction with Nitrous Acid
- Chemical Reactions of Amines - Reaction with Arylsulphonyl Chloride
- Chemical Reactions of Amines - Electrophilic Substitution
- Uses of Amines
- Identification of Primary, Secondary and Tertiary Amines
- Cyanides and Isocyanides
- Introduction of Diazonium Salts
- Method of Preparation of Diazonium Salts
- Physical Properties of Diazonium Salts
- Chemical Reaction of Diazonium Salts - Reactions Involving Displacement of Nitrogen
- Chemical Reaction of Diazonium Salts - Reactions Involving Retention of Diazo Group
- Importance of Diazonium Salts in Synthesis of Aromatic Compounds
- Organic Compounds Containing Nitrogen Numericals
Biomolecules
- Introduction of Carbohydrates
- Classification of Carbohydrates
- Monosaccahrides
- Preparation of Glucose
- Structures of Glucose
- Structure of Fructose
- Disaccharides - Sucrose, Maltose and Lactose
- Polysaccharides - Starch, Cellulose and Glycogen
- Oligosaccharides
- Polysaccharides
- Importance of Carbohydrates
- Introduction of Proteins
- Amino Acids
- Classification of Amino Acids
- Structure of Proteins
- Denaturation of Proteins
- Peptide
- Introduction of Enzymes
- Mechanism of Enzyme Action
- Introduction of Vitamins
- Classification of Vitamins
- Introduction of Nucleic Acids
- Chemical Composition of Nucleic Acids
- Structure of Nucleic Acids
- Biological Functions of Nucleic Acids
- Lipids and Hormones
- Biomolecules Numericals
- Chemical Coordination
Polymers
- Introduction to Polymers
- Classification of Polymers Based on Source
- Classification of Polymers Based on Structure
- Classification of Polymers Based on Mode of Polymerisation
- Classification of Polymers Based on Molecular Forces
- Classification of Polymers Based on Growth Polymerisation
- Types of Polymerisation Reactions - Addition Polymerisation or Chain Growth Polymerisation
- Types of Polymerisation Reactions - Condensation Polymerisation Or Step Growth Polymerisation
- Types of Polymerisation Reactions - Copolymerisation
- Types of Polymerisation Reactions - Rubber
- Molecular Mass of Polymers
- Biodegradable Polymers
- Polymers of Commercial Importance
- Some Important Polymers
- Polymers Numericals
Chemistry in Everyday Life
- Drugs and Their Classification
- Drug-target Interaction - Enzymes as Drug Targets
- Drug-target Interaction - Receptors as Drug Targets
- Therapeutic Action of Different Classes of Drugs - Antacids
- Therapeutic Action of Different Classes of Drugs - Antihistamines
- Therapeutic Action of Different Classes of Drugs - Neurologically Active Drugs
- Therapeutic Action of Different Classes of Drugs - Antimicrobials
- Therapeutic Action of Different Classes of Drugs - Antifertility Drugs
- Chemicals in Food - Artificial Sweetening Agents and Food Preservatives
- Cleansing Agents - Soaps
- Cleansing Agents - Synthetic Detergents
- Chemistry in Everyday Life Numericals
- Corrosion
- Experiment
- Rusting Process and Corrosion Effect
Corrosion:
Redox reactions play a significant role in everyday life, one of the most common examples being the rusting of iron. New vehicles and iron objects appear shiny, but over time, they lose their lustre and develop a reddish-brown layer called rust. The chemical formula of rust is Fe₂O₃·H₂O. This process of rusting is a type of corrosion, which gradually damages metals due to oxidation by air, water, or other environmental factors.
- Rusting is the corrosion of iron when it reacts with moisture and oxygen, forming a reddish-brown layer (rust).
- Corrosion is a general process where metals deteriorate due to oxidation caused by air, moisture, or chemicals. It affects various metals, such as iron, copper, and silver.
- Copper reacts with moist air and carbon dioxide, forming a greenish layer of copper carbonate (CuCO₃), reducing its lustre.
- Silver reacts with hydrogen sulphide (H₂S) in the air, forming black silver sulphide (Ag₂S), tarnishing silver objects.
- Aluminium forms a thin protective layer of aluminium oxide (Al₂O₃), preventing further corrosion.
- Corrosion is a type of redox reaction where metals oxidise in the presence of air, water, or chemicals. In the case of rusting, iron loses electrons (oxidation) and reacts with oxygen and moisture, forming rust.

Rusting
Experiment
1. Aim: To study the conditions necessary for rusting of iron.
2. Requirements: four test tubes, four small iron nails, a rubber cork, boiled water, oil, salt water, and anhydrous calcium chloride.
3. Procedure
- Take four test tubes and place them in a test tube stand.
- In the first test tube, add boiled water and cover it with a layer of oil.
- In the second test tube, add salt water.
- In the third test tube, keep only air.
- In the fourth test tube, place anhydrous calcium chloride and seal it with a rubber cork.
- Put a small iron nail in each test tube and leave them undisturbed for a few days.
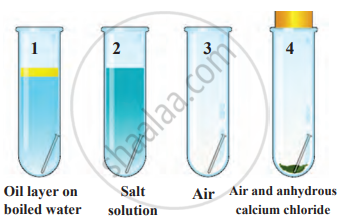
To study rusting
4. Observation
- The nail in the second test tube (salt water) rusted the most.
- The nail in the first test tube (boiled water with oil) did not rust.
- The nail in the third test tube (only air) did not rust.
- The nail in the fourth test tube (with anhydrous calcium chloride) remained unchanged.
5. Conclusion: Rusting occurs only when both air and water are present. The presence of salt (electrolytes) accelerates the rusting process. If either air or water is absent, rusting does not take place.
Rusting Process and Corrosion Effect
The process of rusting of iron occurs through an electrochemical reaction, where different areas of the iron surface act as an anode and cathode. This is demonstrated in the Iron Nail Rusting Experiment under different conditions.
Step 1: Oxidation at the Anode
At the anode, iron loses electrons and forms Fe²⁺ ions.
Fe (s) → Fe²⁺ (aq) + 2e⁻
Step 2: Reduction at the Cathode
At the cathode, oxygen from the air reacts with hydrogen ions (H⁺) and gains electrons to form water.
O₂ (g) + 4H⁺ (aq) + 4e⁻ → 2H₂O (l)
Step 3: Formation of Rust
The Fe²⁺ ions migrate and react with water, getting further oxidised to form Fe³⁺ ions. These Fe³⁺ ions combine with water to form hydrated iron oxide (rust).
2Fe³⁺ (aq) + 4H₂O (l) → Fe₂O₃·H₂O (s) + 6H⁺ (aq)
Effects of corrosion:
Corrosion is a natural process that damages metals over time, leading to
- Weakened metal structures, affecting buildings, bridges, rail transport, ships, etc.
- Economic losses are due to frequent repairs and replacements.
- Safety hazards, as corroded structures may collapse.

Effects of corrosion
