Advertisements
Advertisements
प्रश्न
70 calories of heat are required to raise the temperature of 2 mole of an ideal gas at constant pressure from 30° C to 35° C. The amount of heat required to raise the temperature of the same gas through the same range at constant volume is
विकल्प
30 calories
50 calories
70 calories
90 calories
उत्तर
50 calories
It is given that 70 calories of heat are required to raise the temperature of 2 mole of an ideal gas at constant pressure from 30° C to 35° C. Also, specific heat at constant pressure,
`"C"_"P" = (triangle Q)/(ntriangleT)`
`=> "C"_"P" = 70/ (2 xx (35-30)`
`=> "C"_"P" =70/(2 xx 5)`
`=> "C"_"P" = 7 "calories" -" mol" ^-1K^-1`
For an ideal gas ,
CP - CV = R = 8.314 J - mol -1 K-1 ≃ 2 calories mol-1K-1
⇒ CV =CP -R
⇒ CV = (7-2) calories - mol-1 K-1
⇒ CV = 5 calories - mol-1 K-1
⇒ CV = `(triangle "Q")/(ntriangle"T")`
`=> 5 = (triangle "Q")/(2 xx (35-30)`
⇒ Δ Q = 5 × 2 × (35-30)
⇒ Δ Q = 5 × 2 × 5
⇒ Δ Q = 50 calories
Therefore, 50 calories need to be supplied to raise the temperature of 2 moles of gas from 30-35 oC at constant volume.
APPEARS IN
संबंधित प्रश्न
Estimate the fraction of molecular volume to the actual volume occupied by oxygen gas at STP. Take the diameter of an oxygen molecule to be 3Å.
An air bubble of volume 1.0 cm3 rises from the bottom of a lake 40 m deep at a temperature of 12 °C. To what volume does it grow when it reaches the surface, which is at a temperature of 35 °C?
Consider a collision between an oxygen molecule and a hydrogen molecule in a mixture of oxygen and hydrogen kept at room temperature. Which of the following are possible?
(a) The kinetic energies of both the molecules increase.
(b) The kinetic energies of both the molecules decrease.
(c) kinetic energy of the oxygen molecule increases and that of the hydrogen molecule decreases.
(d) The kinetic energy of the hydrogen molecule increases and that of the oxygen molecule decreases.
Consider a mixture of oxygen and hydrogen kept at room temperature. As compared to a hydrogen molecule an oxygen molecule hits the wall
Calculate the mass of 1 cm3 of oxygen kept at STP.
An electric bulb of volume 250 cc was sealed during manufacturing at a pressure of 10−3 mm of mercury at 27°C. Compute the number of air molecules contained in the bulb. Avogadro constant = 6 × 1023 mol−1, density of mercury = 13600 kg m−3 and g = 10 m s−2.
Use R=8.314J K-1 mol-1
The density of an ideal gas is 1.25 × 10−3 g cm−3 at STP. Calculate the molecular weight of the gas.
Use R=8.31J K-1 mol-1
Consider a sample of oxygen at 300 K. Find the average time taken by a molecule to travel a distance equal to the diameter of the earth.
Use R=8.314 JK-1 mol-1
Estimate the number of collisions per second suffered by a molecule in a sample of hydrogen at STP. The mean free path (average distance covered by a molecule between successive collisions) = 1.38 × 10−5 cm.
Use R = 8.31 JK−1 mol−1
A vertical cylinder of height 100 cm contains air at a constant temperature. The top is closed by a frictionless light piston. The atmospheric pressure is equal to 75 cm of mercury. Mercury is slowly poured over the piston. Find the maximum height of the mercury column that can be put on the piston.
The ratio Cp / Cv for a gas is 1.29. What is the degree of freedom of the molecules of this gas?
Work done by a sample of an ideal gas in a process A is double the work done in another process B. The temperature rises through the same amount in the two processes. If CAand CB be the molar heat capacities for the two processes,
For a solid with a small expansion coefficient,
The value of Cp − Cv is 1.00 R for a gas sample in state A and 1.08 R in state B. Let pAand pB denote the pressures and TA and TB denote the temperatures of the states A and B, respectively. It is most likely that
Let Cv and Cp denote the molar heat capacities of an ideal gas at constant volume and constant pressure respectively. Which of the following is a universal constant?
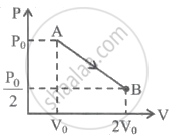
One mole of gas expands obeying the relation as shown in the P-V diagram. The maximum temperature in this process is equal to ______.