Advertisements
Advertisements
प्रश्न
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. Upto which energy level the hydrogen atoms would be excited? Calculate the wavelengths of the first member of Lyman and first member of Balmer series.
उत्तर
Energy of the electron in the nth state of an atom `=-(13.6z^2)/n^2eV`
Here, z is the atomic number of the atom.
For hydrogen atom, z is equal to 1.
Energy required to excite an atom from the initial state (ni) to the final state (nf) = `-13.65/(nf^2)+13.6/n_(i^2)eV`
This energy must be equal to or less than the energy of the incident electron beam.
`:.-13.6/(nf^2)+13.6/n_(i^2)=12.5`
Energy of the electron in the ground state = `-13.6/1^2=-13.6V`
`:.-13.6/nf^2+13.6=12.5`
`=>13.6-12.5=13.6/(nf^2)`
`=>nf^2=13.6/1.1=12.36`
`=>n_f=3.5`
State cannot be a fraction number.∴ nf = 3
Hence, hydrogen atom would be excited up to 3rd energy level.
Rydberg formula for the spectrum of the hydrogen atom is given below:
` 1/lambda=R[1/n_(1^2)-1/n_(2^2)]`
Here, λ is the wavelength and R is the Rydberg constant.
R = 1.097 × 107 m-1
For the first member of the Lyman series:
n1 = 1
n2 = 2
`1/lambda=1.097xx10^7[1/1^2-1/2^2]`
λ = 1215 Å
For the first member of Balmer series:
n1 = 2
n2 = 3
`1/lambda=1.097xx10^7[1/2^2-1/3^2]`
⇒λ = 6563 Å
APPEARS IN
संबंधित प्रश्न
A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom makes a transition from the upper level to the lower level?
A hydrogen atom initially in the ground level absorbs a photon, which excites it to the n = 4 level. Determine the wavelength and frequency of the photon.
The total energy of an electron in the first excited state of the hydrogen atom is about −3.4 eV.
What is the kinetic energy of the electron in this state?
The total energy of an electron in the first excited state of the hydrogen atom is about −3.4 eV.
Which of the answers above would change if the choice of the zero of potential energy is changed?
What are means by pair annihilation? Write a balanced equation for the same.
A 12.9 eV beam of electronic is used to bombard gaseous hydrogen at room temperature. Upto which energy level the hydrogen atoms would be excited ?
Calculate the wavelength of the first member of Paschen series and first member of Balmer series.
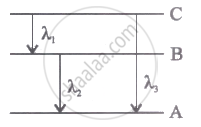
Energy levels A, B, C of acertain atom corresponding to increasing value of energy, i.e., EA< E8 < Ee. If λ1, λ2 and λ3 are the wavelength of radiations corresponding to the transitions C to B, B to A and C to A respectively, which of the following statements is correct?
Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced is ______.
Which of the following is true for X-rays?
The diagram shows the four energy levels of an electron in the Bohr model of the hydrogen atom. Identify the transition in which the emitted photon will have the highest energy.