Advertisements
Advertisements
प्रश्न
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
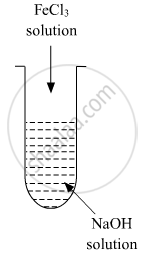
उत्तर
Colloidal particles usually adsorb those ions which are in excess and are common to its own lattice. This preferential adsorption of a particular type of ions imparts a particular type of charge to colloidal particles.
When FeCl3 is added in NaOH, the constituent of the sol is Fe(OH)3 but the dispersion medium is having an excess of OH- ions. Hence, it gets preferentially adsorbed to the sol giving the overall negative charge. The sol is represented as Fe2O3.xH2O / OH-

APPEARS IN
संबंधित प्रश्न
Give reasons for the following observations: Leather gets hardened after tanning.
What happens when persistent dialysis of a colloidal solution is carried out?
Why does leather get hardened after tanning?
What is Gold number?
Gold number is associated with:-
The important factor in the stabilization of a colloidal solution is ______
The right option for the statement “Tyndall effect is exhibited by”, is ______.
Define the following term:
Coagulation
Which of the following is a correct statement?
6.84 g Al2(SO4)3 is needed to coagulate 2.5 L of As2S3 sol completely in 2.0 hrs. The coagulation value of Al2 (SO4)3 is ______.
