Advertisements
Advertisements
प्रश्न
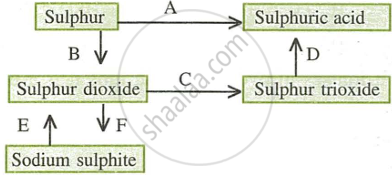
- Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C.
- In the contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead a two-step procedure is used. Write the equations for the two steps involved in D.
- What type of substance will liberate sulphur dioxide from sodium sulphite in step E?
- Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F.
उत्तर
- The catalyst that helps in the conversion of sulphur dioxide to sulphur trioxide in step C is Vanadium pentoxide.
- The two steps for the conversion of sulphur trioxide to sulphuric acid are:
- \[\ce{SO3 + H2SO4 -> H2S2O7}\]
- \[\ce{H2S2O7 + H2O -> 2H2SO4}\]
- The substance that will liberate sulphur dioxide in step E is dilute sulphuric acid \[\ce{H2SO4}\].
- The equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F is:
- \[\ce{SO2 + 2NaOH -> Na2SO3 + H2O}\]
- \[\ce{Na2O + SO2 -> Na2SO3}\]
संबंधित प्रश्न
Give a chemical test to distinguish between dilute sulphuric acid and conc. sulphuric acid.
Name the products formed when hot and concentrated sulphuric acid reacts with Carbon.
Define the following term : Dehydrating agent
Name the following :
The precipitate obtained by treating aqueous lead nitrate with dilute sulphuric acid.
Describe the reaction that show
Concentrated sulphuric acid is a dehydrating agent.
Write the equation for the laboratory preparation of the following salts, using sulphuric acid.
Lead sulphate from lead nitrate.
Write the equation of the following reaction :
Concentrated sulphuric acid is poured over sugar
Write a balanced equation for the following conversion:
Lead sulphate from lead nitrate and sulphuric acid.
The salt is formed when concentrated sulphuric acid reacts with KNO3 above 200°C.
Hydrogen chloride gas is prepared in the laboratory by the action of concentrated sulphuric acid on sodium chloride.
State the method of collection of the gas formed above.
