Advertisements
Advertisements
प्रश्न
A natural linear polymer of 2-methyl-1, 3-butadiene becomes hard on treatment with sulphur between 373 to 415 K and – S – S – bonds are formed between chains. Write the structure of the product of this treatment?
उत्तर
In the manufacture of tyre rubber, 5% of sulphur is used as a crosslinking agent. The probable structures of vulcanised rubber molecules are depicted below:
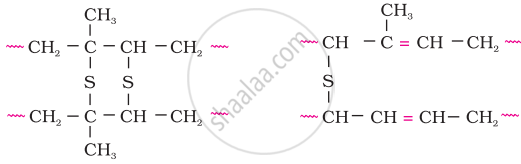
APPEARS IN
संबंधित प्रश्न
Write the names and structures of the monomers of the following polymers: Polyvinyl chloride
Write the name of monomers used for getting the following polymers : Bakelite
Identify the monomer in the following polymeric structures.
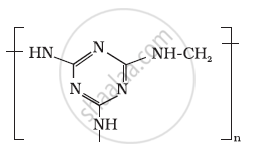
Write a chemical reaction to show that the open structure of D-glucose contains the following :
Straight chain
Identify the type of polymer given in the following figure.

To have practical applications why are cross links required in rubber?
Assertion: Network polymers are thermosetting.
Reason: Network polymers have high molecular mass.
Which of the following is a basic dye?
Which one is disperse dye?
Linear chain polymers have ______.
