Advertisements
Advertisements
प्रश्न
Account for the following :
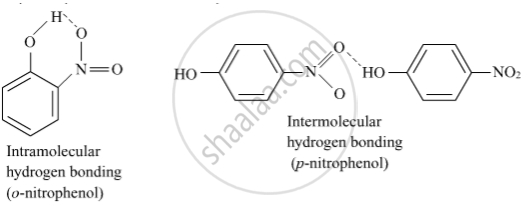
o-nitrophenol is more steam volatile than p-nitrophenol.
उत्तर
o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding while p- nitrophenol is less volatile due to intermolecular hydrogen bonding
APPEARS IN
संबंधित प्रश्न
Ionic solids conduct electricity in molten state but not in solid state. Explain
Sodium crystallizes in bcc structure. If the edge length of unit cell is 4.3 × 10-8 cm, the radius of Na atom is ______.
A compound crystallizes in bcc structure. What is unit cell edge length if diameter of its atom is 120 pm?
Which of the following is TRUE about molecular solids?
Fullerene is an example of ____________.
An example of a covalent crystalline solid is ____________.
In graphite electrons are ____________.
Graphite cannot be classified as ______.
Which of the following cannot be regarded as molecular solid?
(i) SiC (Silicon carbide)
(ii) AlN
(iii) Diamond
(iv) I2
Among the following which is the best description of water in the solid phase?
