Advertisements
Advertisements
प्रश्न
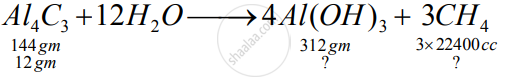
Aluminium carbide reacts with water according to the following equation :
`Al_4C_3 + 12H_2O-> 4Al(OH)_3 + 3CH_4`
1)What mass of aluminium hydroxide is formed from 12g of aluminium carbide?
2) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
[Relatively molecular weight of `Al_4Cl_3 = 144; Al(OH)_3 = 78]`
उत्तर
1)
So, the amount of 3 Al(OH)3 formed will be 26 gm
2) From 12 gm Al4C3 5600 cc methane will be formed
APPEARS IN
संबंधित प्रश्न
Calculate the relative molecular mass of Sodium acetate
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
Calculate the percentage of boron (B) in borax (Na2B4O7.10H2O). [H = 1, B = 11, O = 16, Na = 23],
answer correct to 1 decimal place.
Water can split into hydrogen and oxygen under suitable conditions. The equations representing the change is: 2H2O(I) → 2H2 (g) + O2(g)
Ammonia burns in oxygen and the combustion in the presence of a catalyst may be represented as:
2NH3 (g) +21/2O2 (g) → 2NO (g) + 3H2O (I)
What mass of steam is produced when 1.5 g of nitrogen monoxide is formed?
Washing soda has the formula Na2CO3.10H2O.What is the mass of anhydrous sodium carbonate left when all the water of crystallization is expelled by heating 57.2 g of washing soda?
Calculate the percentage of platinum in ammonium chloroplatinate (NH4)2PtCl6.
[N = 14, H = 1, Pt = 195, Cl =35.5]
(Give your answer correct to the nearest whole number)
Calculate the percentage of sodium in sodium aluminium fluoride (Na3AIF6).
[F = 19, Na = 23, Al = 27]
Calculate the volume of oxygen required for the complete combustion of 8.8 g of propane (C3H5).
(Atomic mass: C = 14, O = 16, H = 1, Molar Volume = 22.4 dm3 at STP.)
Calculate the relative molecular mass of:
Potassium chlorate
Calculate the relative molecular mass of:
CHCl3
Calculate the relative molecular mass of:
(NH4)2 Cr2O7
