Advertisements
Advertisements
प्रश्न
Aluminium carbide reacts with water according to the following equation :
`Al_4C_3 + 12H_2O-> 4Al(OH)_3 + 3CH_4`
1)What mass of aluminium hydroxide is formed from 12g of aluminium carbide?
2) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
[Relatively molecular weight of `Al_4Cl_3 = 144; Al(OH)_3 = 78]`
उत्तर
1)
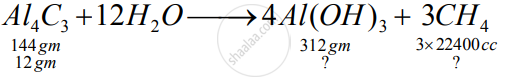
So, the amount of 3 Al(OH)3 formed will be 26 gm
2) From 12 gm Al4C3 5600 cc methane will be formed
APPEARS IN
संबंधित प्रश्न
An organic compound has the following percentage composition: C = 12.76%, H = 2.13%, Br = 85.11%. The vapour density of the compound is 94. Find out its molecular formula.
Find the total percentage of oxygen in magnesium nitrate crystal Mg(NO3)2.6H2O.
[H = 1, N = 14, O = 16, Mg = 24]
Mention the term defined by the following sentence:
The mass of a given volume of gas compared to the mass of an equal volume of hydrogen.
A flask contains 3.2g of sulphur dioxide. Calculate the following: The number of molecules of sulphur dioxide present in the flask.
Calculate the volume of oxygen required for the complete combustion of 8.8 g of propane (C3H5).
(Atomic mass: C = 14, O = 16, H = 1, Molar Volume = 22.4 dm3 at STP.)
Give one word or phrase for the following:
The ratio of the mass of a certain volume of gas to the mass of an equal volume of hydrogen under the same conditions of temperature and pressure.
Complete the following calculations. Show working for complete credit :
If the empirical formula of a compound is CH and it has a vapour density of 13, find the molecular formula of the compound.
A gaseous hydrocarbon contains 82.76% of carbon. Given that its vapour density is 29, find its molecular formula.
[C = 12, H = 1]
Calculate the relative molecular mass of:
Ammonium chloroplatinate (NH4)2 PtCl6
Which of the following contains maximum number of molecules?
