Advertisements
Advertisements
प्रश्न
An element A has 2 electrons in its fourth shell. State:
position in the periodic table
उत्तर
Position in the periodic table is group 2 period 4
APPEARS IN
संबंधित प्रश्न
The electrons in the atoms of four elements A, B, C and D are distributed in three shells having 1, 3, 5 and 7 electrons in the outermost shell respectively. State the period in which these elements can be placed in the modern periodic table. Write the electronic configuration of the atoms of A and D and the molecular formula of the compound formed when A and D combine.
What is its atomic number?
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 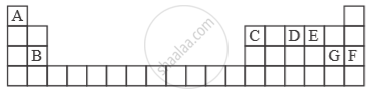
Which is an inert gas?
How many elements are there in period 2?
Arrange the following in order of increasing radii:
Mg2+, Mg, Mg+
State the nature of compounds formed when group 17 elements combine with (i) metals (ii) non-metals.
Moving down in the second group, number of valence electrons ______.
The electronic configuration of an element is 2, 8, 4. State it:
group and period in the Modern Periodic Table.
Write the electronic configuration of two elements A and B whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element A reacts with element B. State whether this compound is acidic, basic or neutral. Give a reason to justify your answer.
Chlorine in the Periodic Table is surrounded by the elements with atomic number 9, 16, 18 and 35.
Which of these have Physical and Chemical properties resembling chlorine?
