Advertisements
Advertisements
प्रश्न
Study the extract of the Periodic Table given below and answer the questions that follow. Give the alphabet corresponding to the element in question. DO NOT repeat an element. 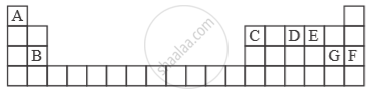
Which is an inert gas?
उत्तर
Inert gas - ‘F’.
APPEARS IN
संबंधित प्रश्न
The elements 4Be, 12Mg and 20Ca, each having two valence electrons in their valence shells, are in periods 2, 3 and 4 respectively of the modern periodic table. Answer the following questions associated with these elements, giving reason in each case:
(a) In which group should they be?
(b) Which one of them is least reactive?
(c) Which one of them has the largest atomic size?
Consider two elements 'A' (Atomic number 17) and 'B' (Atomic number 19) :
(i) Write the positions of these elements in the modern periodic table giving justification.
(ii) Write the formula of the compound formed when 'A' combines with 'B.'
(iii) Draw the electron dot structure of the compound and state the nature of the bond formed between the two elements.
How does the tendency to lose electrons change as we go down in group 1 of the periodic table? Why does it change this way?
Which one of the following does not increase while moving down the group of the periodic table?
What is its atomic number?
Why fluorine has higher E.N. than chorine?
Name the elements in period 1.
Copy and complete the following sentence choosing the correct word or words from those given below, at the end of the sentence:
The similarities in the properties of elements belonging to a group are because they have the same ______
What is meant by a Group in the Periodic Table?
Fill in the blanks from the words A to F given below.
A: Decreases
B: Increases
C: Remains same
D: Increases by one
E: Electropositive
F: Electronegative
Down a group in the Modern Periodic Table.
No. of electron shells __________; No. of valence electrons ________; Electronegativity ________ Character of elements changes from _________ to ____________.
