Advertisements
Advertisements
प्रश्न
Answer the following question:
What is the universal indicator? Does Mg (OH)2 react with sodium hydroxide? If not, why?
उत्तर
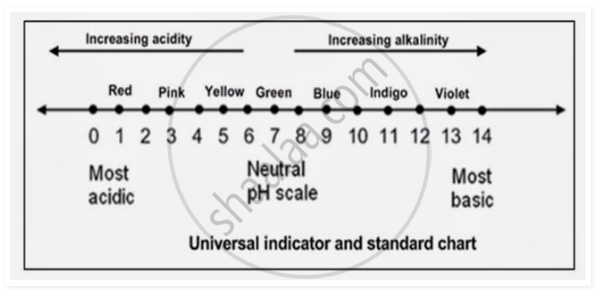
- Universal indicator is an indicator which is a mixture of several indicators.
- It has a very wide range and can indicate pH value almost from 0 to 14.
- When one or two drops of the universal indicator are added to a solution, it changes its colour. This colour is matched against the colour on a standard colour chart (usually given on its bottle) to find the pH, and the pH value can be read. This indicates the strength of the solution too.
- Universal indicator is available as a solution or in the form of paper strips.
- Mg(OH)2 does not react with sodium hydroxide as both are bases.
APPEARS IN
संबंधित प्रश्न
Why does an aqueous solution of an acid conduct electricity?
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute sulphuric acid reacts with aluminium powder.
Complete and balance the following chemical equations:
Zn (s) + HCI (aq) →
Hydrochloric acid reacts with a metal X to form a gas Y which burns with a 'pop' sound. Sodium hydroxide solution also reacts with the same metal X (on heating) to form the same gas Y.
Write the chemical equation of the reaction of metal X with (i) hydrochloric acid, and (ii) sodium hydroxide solution.
Write the chemical formula of soda ash?
State one use of bleaching powder (other than bleaching).
State two uses each of the following compounds:
Sodium hydroxide
Is PbO2 a base or not? Comment.
What are esters? How are esters prepared? Write the chemical equation for the reaction involved. What happens when an ester reacts with sodium hydroxide? Write the chemical equation for the reaction and also state the name and use of this reaction.
If you take some distilled water in a test-tube, add an equal amount of acetic acid to it, shake the test-tube well and leave it undisturbed on the test-tube stand, then after about 5 minutes what would you observe?
(A) There is a layer of water over the layer of acetic acid.
(B) A precipitate is setting at the bottom of the test-tube.
(C) Bubbles of colourless gas are coming out of the test-tube.
(D) There is a clear, colourless transparent solutions in the test-tube.
Write the chemical formula of washing soda. How can it be obtained from baking soda? List two industries in which washing soda is used for other purposes than washing clothes.
A solution of NaCl
(i) will turn red litmus blue
(ii) will turn pH paper green
(iii) will turn blue litmus red
(iv) will not affect litmus
Lime water reacts with chlorine to form:
Common salt besides being used in kitchen can also be used as the raw material for making
- washing soda
- bleaching powder
- baking soda
- slaked lime
What happens when nitric acid is added to egg shell?
Can we taste acids and bases to identify them?
The formula of bleaching powder is ______.
For each of the salt: A, B, C and D, suggest a suitable method of its preparation.
C is a soluble salt of copper.
Give a balanced equation for the reaction:
Silver nitrate solution and sodium chloride solution.
