Advertisements
Advertisements
Question
Answer the following question:
What is the universal indicator? Does Mg (OH)2 react with sodium hydroxide? If not, why?
Solution
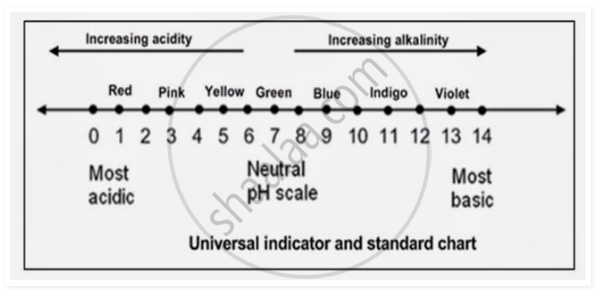
- Universal indicator is an indicator which is a mixture of several indicators.
- It has a very wide range and can indicate pH value almost from 0 to 14.
- When one or two drops of the universal indicator are added to a solution, it changes its colour. This colour is matched against the colour on a standard colour chart (usually given on its bottle) to find the pH, and the pH value can be read. This indicates the strength of the solution too.
- Universal indicator is available as a solution or in the form of paper strips.
- Mg(OH)2 does not react with sodium hydroxide as both are bases.
APPEARS IN
RELATED QUESTIONS
How is the concentration of hydroxide ions (OH−) affected when excess base is dissolved in a solution of sodium hydroxide?
A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains ______.
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute sulphuric acid reacts with zinc granules.
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute sulphuric acid reacts with aluminium powder.
Fill in the blank in the following sentences:
Substances do not show their acidic properties without.......................... .
What happens when an acid reacts with a metal oxide? Explain with the help of an example. Write a balanced equation for the reaction involved.
On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
On the basis of the above reaction, what can you say about the nature of copper oxide?
What ions are present in the solutions of following substances? (write the symbols only)
Hydrochloric acid
What ions are present in the solutions of following substances? (write the symbols only)
Magnesium hydroxide
Hydrochloric acid reacts with a metal X to form a gas Y which burns with a 'pop' sound. Sodium hydroxide solution also reacts with the same metal X (on heating) to form the same gas Y.
Write the chemical equation of the reaction of metal X with (i) hydrochloric acid, and (ii) sodium hydroxide solution.
Complete and balance the following chemical equations:
`Ca(OH)_2 + Cl_2 ->`
What happens when a solution of sodium hydrogencarbonate is heated? Write equation of the reaction involved.
What are esters? How are esters prepared? Write the chemical equation for the reaction involved. What happens when an ester reacts with sodium hydroxide? Write the chemical equation for the reaction and also state the name and use of this reaction.
Explain the following:
Lead carbonate does not react with dilute HCl.
Write any four physical properties of acids.
Common salt besides being used in kitchen can also be used as the raw material for making
- washing soda
- bleaching powder
- baking soda
- slaked lime
Which of the following statements is true for acids?
A sulphate salt of Group 2 element of the Periodic Table is a white, soft substance which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purposes. Identify the sulphate salt. Why does it show such a behaviour? Give the reaction involved.
A salt may be ______.
______ change the colour of the indicators.
